UK Pharmaceutical company Merck Serono has today announced that its investigational drug L-BLP25, for locally advanced stage IIIA or IIIB non-small cell lung cancer, has failed to meet the primary endpoint in its START Phase III trial.
The endpoint of the Phase III study was to demonstrate a statistically significant improvement in overall survival.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
However, despite not meeting expectations, notable treatment effects were seen for L-BLP25 in certain sub-groups.
Further analyses are planned in the next few weeks to explore the potential benefit-risk profile of L-BLP25 in certain populations.
The data will then be discussed with external experts and regulatory authorities.
The START trial was a randomised, multicenter, double-blind, placebo-controlled trial that assessed the efficacy, safety and tolerability of L-BLP25 in more than 1,500 patients with unresectable stage III NSCLC who had achieved response or stable disease after chemoradiotherapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPrincess Margaret Hospital Lung Cancer Research Scott Taylor chair, University of Toronto professor of medicine and trial coordinating investigator Dr Frances Shepherd said; "It is disappointing that the START trial did not meet its primary endpoint, in particular for patients suffering from NSCLC.
"However, notable treatment effects were observed in certain subgroups of patients and warrant further investigation of L-BLP25."
L-BLP25 is an investigational MUC1 antigen-specific cancer immunotherapy that is designed to stimulate the body’s immune system to identify and target cells expressing the cell surface glycoprotein MUC1.
MUC1 is expressed in many cancers, such as non-small cell lung cancer (NSCLC), and has multiple roles in promoting tumor growth and survival.
L-BLP25 is currently being tested in another trial called INSPIRE, also for the treatment of unresectable stage III NSCLC.
The INSPIRE study is a Phase III, multi-center, randomised, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy, safety and tolerability of L-BLP25 in patients of Asian heritage suffering from unresectable, stage IIIA or IIIB NSCLC who have had a response or stable disease after at least two cycles of platinum-based chemoradiotherapy.
Merck Serono Global Drug Development and Medical head Dr Annalisa Jenkins said; "We believe that the START study will offer important scientific insights to the potential for immunotherapies in the treatment of this devastating disease and we intend to discuss these data with scientific community and regulatory authorities to gain their advice on potential next steps."
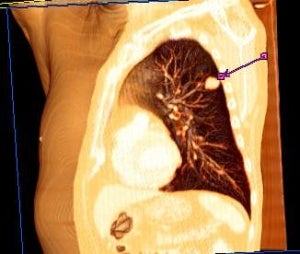
Image: The study was to check the efficacy, safety and tolerability of L-BLP25 in patients with a form of lung cancer. Photo: Courtesy of Lange123.




