
In a significant move that could reshape how pharmaceutical companies conduct CNS clinical trials, Cambridge Cognition has unveiled in-house rater training service that promises to transform the accuracy and efficiency of clinical assessments.
A challenge that has long plagued neuroscience research stands true today: how to ensure that the people evaluating patients in clinical trials – known as raters – do so consistently and accurately in a time effective way. It’s a crucial aspect of brain health research that can make or break a clinical trial’s success. In 2023, the CNS clinical trials market had estimated revenues of $5.2bn. The services segment comprised more than half of the total revenue. In the next five years, the market has an estimated annual growth rate more than 27%. Furthermore, it is vital that raters are trained to the necessary levels to avoid errors – which can lead to failure of clinical trials with significant costs incurred.
Cambridge Cognition, a leading innovator in brain health technology, has been advancing its solutions by leveraging its deep expertise and market-leading voice capabilities. This evolution has been further strengthened by its acquisition of Winterlight Labs in 2023.
“Imagine having an expert by your side – one that never tires, swiftly identifies details a human might miss, and enhances the precision of clinical assessments,” explains Rob Baker, the company’s Joint Managing Director. This is the essence of their AQUA technology, which automates the review of audio recordings from clinical assessments, flagging errors traditionally identified manually. “By streamlining the initial review process, AQUA allows human reviewers to focus on areas that truly matter, optimizing both time and cost efficiency. Technology should work alongside human expertise, not just as a tool we directly use, but as a helpful background support that improves our interactions and helps us achieve more reliable results.” This approach reflects the company’s commitment to technologies that complement human expertise.
The synergy between AQUA’s automated quality assurance and comprehensive rater training creates a powerful feedback loop that elevates the quality of clinical assessments. While rater training establishes the foundation for standardized evaluations, AQUA continuously monitors and identifies areas where additional training may be beneficial. This [real-time] insight allows for targeted training interventions, ensuring raters maintain high standards throughout the trial. When pharmaceutical companies deploy both solutions together, they see measurable improvements in data quality, reduced variability in assessments, and significant time savings in quality control processes. The integrated approach has proven particularly valuable in complex CNS trials where subtle changes in patient responses can have significant implications for trial outcomes.
The timing of this development is particularly interesting. The pharmaceutical industry is undergoing a significant shift, moving away from paper-based systems that have dominated for decades. While these traditional methods still account for nearly half of all assessments, there’s a growing recognition that they’re no longer sufficient for modern research needs. According to GlobalData’s Clinical Trial database, just under 200 clinical trials recorded featured a virtual component in 2016. However, the number of clinical trials using a virtual component in 2024 reached almost 450.
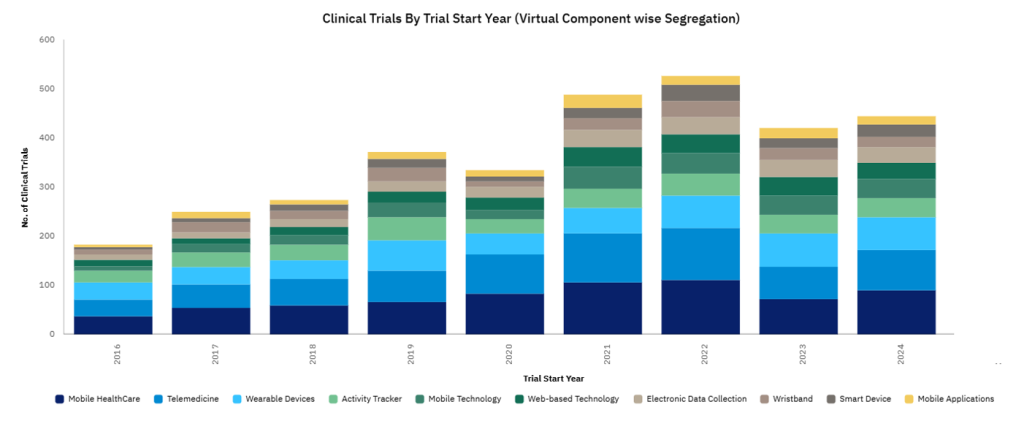
As clinical trials become more complex and data-driven, the tools used to conduct them must evolve. Cambridge Cognition’s platform, which combines training for human raters with advanced technology, represents this evolution in-action.
Several pharmaceutical companies have already begun incorporating this new approach into their upcoming trials for 2025. But perhaps more importantly, it signals a shift in how we think about clinical research – moving from isolated tools and processes to more integrated, technological solutions.
Rob Baker, the company’s Joint Managing Director, finalised: “In research, consistency is everything. The more we can reduce human variability while maintaining the human touch, the closer we get to understanding the complexities of the brain.” It’s a balance that may well define the future of neuroscience research.
An integrated, all-in-one platform, the Cambridge Cognition eCOA solution is already being utilised by many top pharma companies seeking accuracy, efficiency, and reliability in their clinical trials. To learn more, download the report below.


