
As clinical research continues evolving to become more innovative and efficient, adaptive trials, specifically platform trials, are leading the charge.
Platform trials enable the simultaneous study of multiple treatments against a signal control group within the same overall trial structure. Inherently adaptive, platform trials can be modified to add or drop treatments over time based on interim results, making them a more efficient and flexible option compared with standard clinical trial models.
This multifaceted approach allows researchers to evaluate different therapies for a single disease at the same time, offering useful opportunities for comparison and contrast.
The race to find effective therapies for Covid-19 created a spike in popularity for platform trials, with the most in 2020 and 2021 according to GlobalData’s Clinical Trials Database. While 2022 saw a reduction of 53% in adaptive trials, they rebounded by 75% in 2023 to almost match pandemic levels, indicating there is still ongoing interest in this clinical trial method beyond the pandemic.
Because platform trials can test a combination of therapies, there is greater potential for participants to be randomized in an active treatment arm and, consequently, receive additional benefits. This can lead to enhanced engagement from participants and fewer dropping out of the study.
But while platform trials have the potential to be more effective and less expensive than traditional trials, challenges remain concerning data management. Integrated cloud-based electronic data capture (EDC) solutions are providing clinicians with the tools they need to harness the full potential of platform trial benefits.
This article explores the benefits of adaptive trials and how unified EDC platforms can best leverage these benefits to overcome complex study builds.
Platform trials explained
Adaptive trials, including platform trials, are clinical studies designed for flexibility, allowing certain aspects of the trial to be modified as data is collected. Unlike traditional trials that follow a fixed protocol, adaptive trials can introduce changes such as adjusting doses, adding or dropping treatment arms, or modifying sample sizes.
With platform trials, the goal is to design a master protocol that outlines the common elements of the trial which will remain constant along with the mechanisms that allow for adaptation. This approach maximizes the utility of the trial, ensuring resources are allocated efficiently and offers the promise of a better experience for its participant.
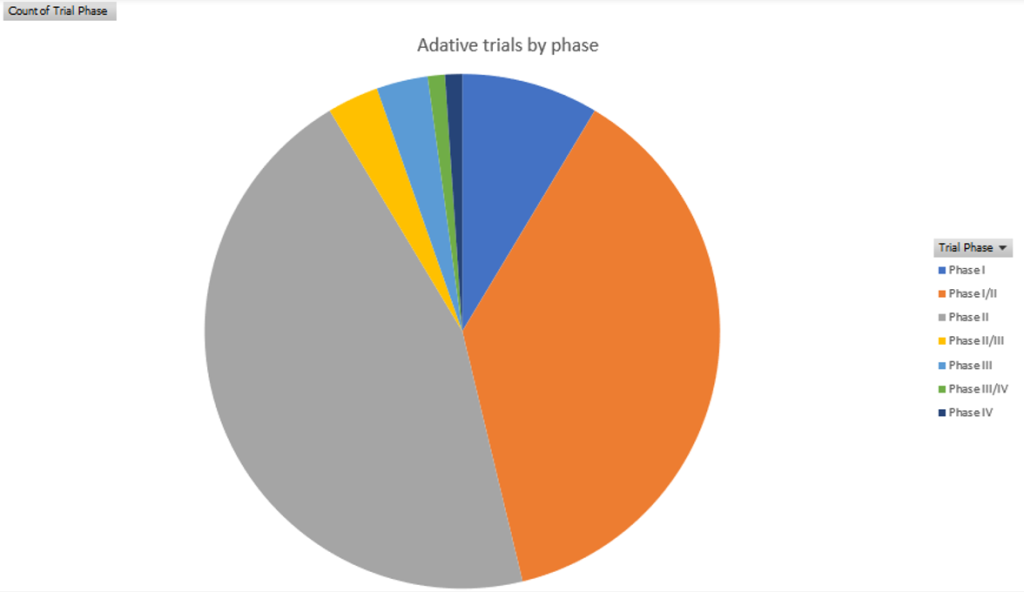
Adaptive trials are increasingly being used across all phases of clinical trials. Out of the adaptive trials recorded by GlobalData running globally since 2015, the most were in Phase II at 45.6%, followed by 38% in Phase I-II.
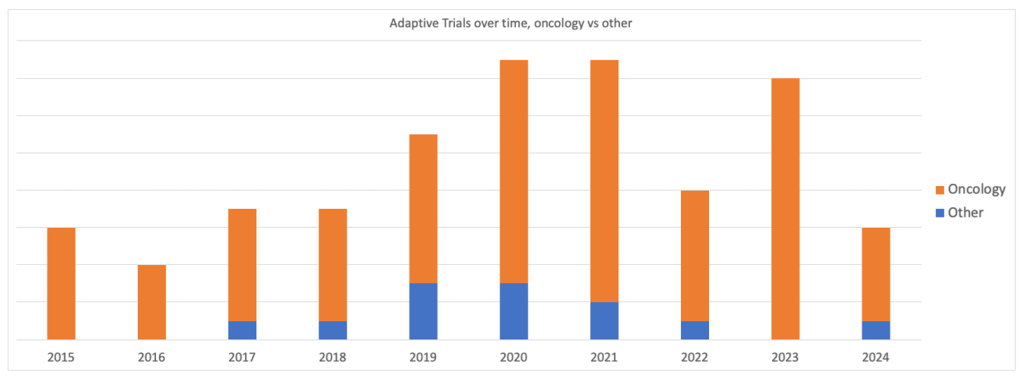
According to GlobalData intelligence, oncology has been the dominant field for platform trials since 2015. This trend did not change in the peak pandemic years of 2020 and 2021.
“In platform trials, it’s essential to adapt based on how participants respond to treatments,” explains Bradham. “This requires incorporating specific measurement criteria into the protocol, such as biomarkers, additional treatment cycles, or adjusted treatment combinations. The principles of precision medicine align well with this approach, allowing platform trials to be designed with flexibility from the outset.”
Platform trials help consolidate resources, reduce inefficiencies, improve patient experience
A major source of inefficiency in clinical trials is that multiple therapies and sponsors are often involved in separate trials in the same therapeutic area. Platform trials provide a key advantage on this front, by combining findings from multiple studies to improve outcomes for stakeholders.
This function also extends to combining resources to reduce operational inefficiencies in how the trial is conducted. Processes can be taken under one umbrella, from designing a unifying protocol to recruiting sites and participants. By spreading tasks across different stakeholders, platform trials reduce costs and minimize challenges such as finding patients, maintaining the control group, and regulatory burdens.
”Running individual trials can be costly for sponsors and biotechs,” notes Bradham. “Platform trials offer a more efficient solution, enabling CROs to conduct trials effectively, optimize resources, and ultimately pass cost savings back to the sponsors and biotechs.”
Beyond just consolidating resources and reducing cost inefficiencies for the sponsors and biotechs running the study, platform trials can also improve the experience for patients. Some patients participate in trials because they are looking for an alternative to standard care, and can end up disappointed when that expectation is not followed through on.
However, in a platform trial where multiple therapies are being tested, the likelihood of participants being randomized into one of the active treatment arms is higher. The benefits for study participants are therefore greater, resulting in improved patient engagement and lower study dropout rates.
A real-world case study of adaptive trials
A leader in the EDC space, Zelta by Merative is a cloud-based unified data management system and acquisition platform with customizable modules. Zelta’s functionalities can be tailored to support even the most complex clinical trial demands, including platform trials.
During the pandemic, Zelta partnered with several trials investigating therapies for Covid-19. One platform study included four different treatment arms in three countries and 30,000 study participants over two and a half years. In a trial of this scale and complexity, flexibility was paramount to ensure success.
More than 20 post-go-live changes were implemented through the Zelta platform in the study. Ordinarily, making one post-go-live change to a trial model can take days to update and lead to downtime in data collection. However, with Zelta, the timeframe for implementing updates is shortened substantially.
“On average, it took about 18 minutes to implement these changes, significantly reducing the disruption to data collection,” adds Bradham. “Typically, deploying post-go-live changes can take days, during which the system is offline, preventing data collection, report generation, and analysis.
“This efficiency is crucial when managing tens of thousands of participants. Otherwise, sites face the additional burden of maintaining data and coordinating its entry once the system is back online. According to a study by the Tufts Center for the Study of Drug Development, each mid-study update can add approximately 30 days to clinical trial timelines.”
How Zelta supports adaptive trials
The efficiency of the Zelta platform helped expedite timelines in the critical race to better manage the extra demands from the Covid-19 pandemic.
“In a platform trial, when you have different sponsors and stakeholders, having a cloud-based solution can dramatically simplify that, and take a lot variables and vendor management off the table,” says Bradham.
In the Zelta platform, all data is available without needing to be integrated or programmed separately to involve different teams or vendors. By hosting randomization and trial supply management, medical coding, and lab management on a singular platform, Zelta can help simplify the platform trials process.
From a proprietary perspective, Zelta can also separate data from different sponsors and biotechs into their datasets to ensure information is being correctly extracted and received by the right personnel.
Offering a unified approach to digital clinical trial management and enhancing data accessibility, Zelta is a high-performing, adaptable solution that can streamline even the most complex clinical trials.
To learn more about how Zelta and its adaptive trial support can help you meet your clinical trial goals, download the report below.
Click here to read more exclusive Zelta content.



