
Temperature sensitivity is a primary concern for many parenteral drug products. Certain medications require cold storage, freezing, or lyophilization to manage this sensitivity. However, a critical challenge arises: how do manufacturers produce these products in cleanrooms and filling lines that are at controlled room temperature?
This article explores the strategies that fill finish CDMOs use to effectively manage temperature-sensitive drug products during the fill finish process.
Understanding the sensitivity
The first step to designing an effective process requires understanding the drug product and its limitations. Stability data – even from a short-term hold time study – will be critical in determining what modifications are necessary. Some temperature-sensitive drug products will remain within its target profile for weeks at room temperature and do not require any process change or modifications to effectively manufacture. Other products will fall out of acceptable ranges within a few days at room temperature.
The first step a CDMO will take to design the process is to gather stability data. If none exists already, then a short hold study will be performed to advise the fill finish process.
Keeping the product cool
The cleanrooms and filling lines where drug product is formulated and filled remains at controlled room temperature. If the drug product is exceptionally temperature sensitive and cannot remain at room temperature for the duration of the fill finish process, then jacketed mixing vessels may be employed to limit heat exposure (see Figure 1). Cool water, controlled to within 6 – 15°C, circulates between the water jacket and a water bath. The drug product solution is formulated in and filled from a vessel with a jacket to keep the product cool throughout the process. There are also special jackets to cool bioprocess bags (i.e. biobags), if this type of container is preferred for filling.
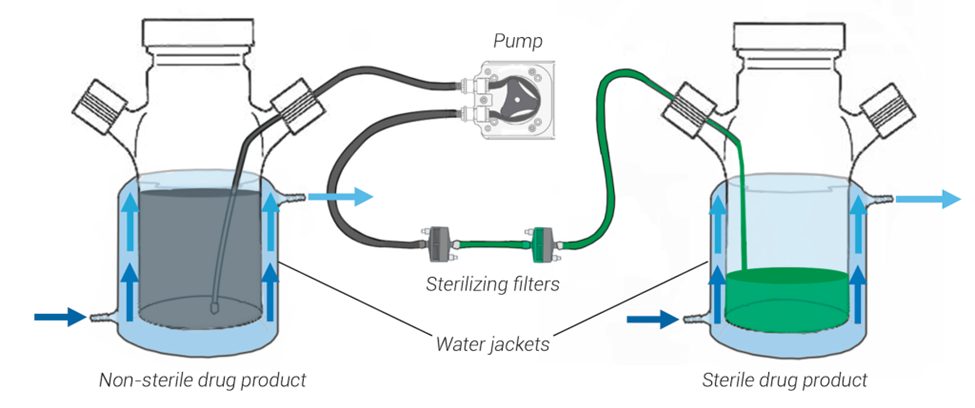
Figure 1. A sterile filtration setup using jacketed mixing vessels. Water circulates between the water jacket and a water bath to maintain the temperature of the jacket within a set range. This process is effective at keeping the drug product cool during formulation and filling activities.
Outside of using jacketed vessels, the best approach is to keep the drug product in a temperature-controlled unit (TCU) when possible. If the API is a frozen powder, then this is removed directly from the freezer before formulation activities take place. If the API is a frozen solution or the drug product is already formulated and arrives frozen, then this should be thawed directly before formulation and filtration activities in a cold, non-frozen TCU (such as a 2 – 8°C TCU). Some products may require an operator to invert the product at regular intervals to safely speed the thawing process.
If there’s a considerable wait between process steps, the bulk drug product should be removed to a cold, non-frozen, TCU. For example, drug product should be moved to a 2 – 8°C TCU after formulation and before filling activities begin if these occur on different days.
Moving quickly
Time is the enemy of stability, especially for temperature-sensitive drug product. Anything that can be done to safely speed the fill finish process should be implemented.
When receiving the drug substance or drug product, the item should be unpacked, inspected, and stored immediately. For example, at Berkshire Sterile (now Sharp Sterile Manufacturing), these shipments are scheduled, allowing the product to be inspected and moved into the appropriate temperature-controlled unit (TCU) within ten minutes of receipt.
Frozen solutions that need to be thawed for formulation or sterile filtration activities may be gently inverted at regular intervals to safely speed thawing.
After filling, the drug product remains in a cold, non-frozen TCU until a thorough 100% visual inspection of the lot is completed. Some products that require frozen storage may undergo expedited inspection, allowing them to be prioritized and moved into the freezer sooner. In very extreme cases, an “off the line” inspection can be performed – where product is removed from the filling line and immediately brought to the visual inspection suite. This allows visual inspection to occur alongside sterile filling operations, reducing the amount of time the drug product remains out of the freezer.
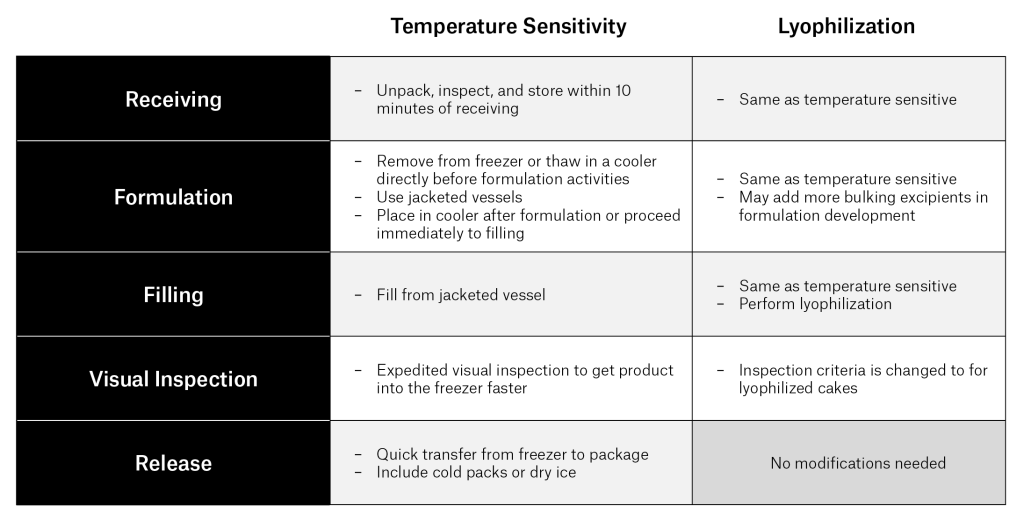
Table 1. Approaches for managing temperature-sensitive drug product and lyophilized drug product at each step of the fill finish process.
Lyophilization
Some drugs are incredibly sensitive, prompting the use of lyophilization, also known as freeze-drying, to bolster product stability and reduce storage and distribution risks. The fill finish process for lyophilized drugs typically mirrors that of temperature-sensitive products until it enters the lyophilizer. Once lyophilized, the product’s stability is greatly improved.
Lyophilization presents unique challenges. Cake quality is critical and often requires some formulation development or lyo cycle development to optimize. In addition, the fill finish process takes longer, requires special equipment, and costs more. Despite these challenges, lyophilization is worthwhile for certain drugs due to the substantial stability benefits it offers.
Adjusting the formulation
If the drug product cannot be managed effectively with the strategies discussed in this article, the next likely step is to adjust the formulation. This may involve altering excipients, adding stabilizers, adjusting the pH, or modifying the API concentration and fill volume to enhance stability. Conducting a rapid hold time study in the lab will likely be enough to identify areas of improvement.
Conclusion
Temperature-sensitive drug products can be effectively managed during fill finish operations with a few fundamental strategies: keeping the product cool and moving quickly. If these approaches are not enough, then the drug product may require lyophilization or formulation development. Planning, executing quickly, and refining the formulation and fill finish process is key to manufacturing safe and effective drug products and maintaining their potency and efficacy despite their sensitivity.
About Sharp’s services
Sharp is a leader in pharmaceutical packaging, clinical trial supply services and small-scale sterile manufacturing. For 70-plus years, we’ve provided solutions to pharma and biotech clients from Phase I trials through to commercial launch and lifecycle management. With facilities in the United States, United Kingdom, Belgium and the Netherlands and more than 30 clinical depots globally, covering every region of the world, our experience is your strength.



