Immunotherapeutics have transformed the landscape of cancer outcomes. It is crucial that the atypical response patterns seen with them are understood and assessed accurately to the extent possible so that no patient is deprived of a delayed favourable outcome. Diligence and rigor are required to ensure the highest standards of data accuracy and integrity for validation of morphological variants of RECIST criteria recommended for response evaluation of immunotherapies.
Patterns of responses to immunotherapies
In response to systemic intervention, tumours may not always shrink in size – at least not initially. Necrosis, infarction, haemorrhage, cavitation, intracellular and vasogenic edema or inflammation and immune cell infiltration into the tumour can all cause them to enlarge or limit size reduction. In fact, a reducing tumour metabolism in the FDG PET-CT implying viable tumour cell reduction may not always translate to a change in diametric size at the same time; it may take longer to manifest.
Atypical patterns of response are more frequently seen with immunotherapies, requiring stronger response assessments. For immunotherapies, the morphological response patterns extend beyond those of cytotoxic agents and include pseudoprogression, durable responses, hyperprogression and dissociated responses.
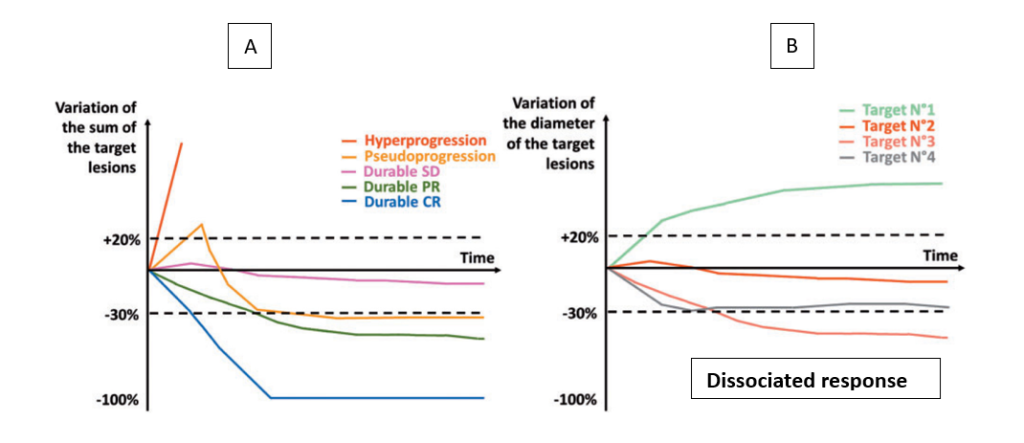
- Pseudoprogression is defined as an illusory increase in tumour burden and is quite rare (<10% overall). Patients can present with either an initial increase in size of the known lesions followed by a subsequent response or newly detectable (previously occult) lesions being diagnosed after start of the immunotherapy that subsequently responded to therapy. [2]
- Durable responses: Immunotherapies have induced long-lasting remission (>5 years) in melanomas, though this success hasn’t been replicated in other cancers and far fewer than half of patients have durable outcomes with immunotherapy, even in combination. Still, meta-analysis including 19 studies showed a proportion of durable responses in 25% of the patients treated with Immune Checkpoint Inhibitors (ICIs), which is 2.3 times higher than of those treated without ICIs (11%). [3]
- Hyperprogressive disease is a paradoxical acceleration of tumour growth kinetics after the initiation of treatment with anti-PD-1/PD-L1 agents, but it has also been reported in patients receiving other treatments. The incidence of hyperprogressive disease observed across different solid tumour types is variable (~10%). It was first reported in retrospective studies of patients treated with ICIs based on clinical observations of patients whose disease seemed to grow faster after the initiation of immunotherapy. [4]
- Dissociated response is considered a mixed radiological or heterogeneous response pattern when responding and nonresponding lesions or new lesions coexist simultaneously. The rate of DR reported in different studies ranges from 3.3–47.8% due to diverse definition and time trends of assessment. A combination analysis showed a seemingly significant difference regarding the frequency ofDR in different types of solidcancer (p = 0.0001) with a 30.3% rate in RCC, 14.3% in endometrial carcinoma, 13.2% in NSCLC and 12.5% in mesothelioma. [5]
Clinical implications for atypical responses
| Pseudoprogression | Durable responses | Hyperprogression | Dissociated responses |
|---|---|---|---|
| Such scenarios would be classified as PD by RECIST v1.1 criteria and would result in end of study for the patients, depriving them of potential benefit later. Favourable prognosis* implies the need to identify pseudoprogressors and ensure continued treatment. |
Sustained responses raise the clinical questions on optimal duration of therapy; until progressive disease occurs or until a response occurs. This may also pose clinical dilemma on sequencing of agents when a possibility of delayed response is foreseen. | Hyperprogression should not have to wait until the first imaging as effective salvage therapy must be instituted based on earlier prediction tools. It is correlated with worse survival in several studies. | A detailed lesion-by-lesion analysis is required to capture this pattern. A potentially favourable prognosis* implies that combination with localised radiotherapy/ intratumoural/ hyperthermic approaches be evaluated where available and preferably continued on treatment. |
*European Journal of Cancer Volume 167, May 2022, pages 42-53. https://doi.org/10.1016/j.ejca.2022.02.024
Assessing alternative morphologic responses
Five morphologic criteria (Figure 2) are now used to assess changes in target lesion sizes, taking into account the specific response patterns after immunotherapy. These include: immune-related response criteria (irRC), immune-related RECIST (irRECIST), immune RECIST (iRECIST), immune-modified RECIST (imRECIST), and lastly, intratumoural RECIST (itRECIST) to be used when evaluating localised immunotherapy approaches. [6]
| Lesion definition | CR | PR | SD | PD | Confirmation of PD | New lesions |
|---|---|---|---|---|---|---|
| RECIST 1.1, 2009 Uni-dimensional ≥10mm, 5 lesions, 2/organ |
Disappearance of lesion | ≥30% decrease from baseline | Neither CR nor PD | ≥20% & increase from the nadir (≥5mm) | Not applicable | PD |
| irRC, 2009 Bi-dimensional 5×5mm 15 lesions, 5/organ |
Disappearance of lesion | ≥50% decrease from baseline | Neither CR nor PD | ≥25% increase from the nadir | At least 4 weeks | Incorporated to the sum of measurement |
| irRECIST, 2013 Uni-dimensional ≥10mm, 5 lesions, 2/organ |
Disappearance of lesion | ≥30% decrease from baseline | Neither CR nor PD | ≥20% increase from the nadir (≥5mm) | 4-12 weeks | Incorporated to the sum of measurement |
| iRECIST, 2017 Uni-dimensional ≥10mm, 5 lesions, 2/organ |
Disappearance of lesion | ≥30% decrease from baseline | Neither CR nor PD | ≥20% increase from the nadir (≥5mm) | 4-8 weeks | iUPD |
| imRECIST, 2018 Uni-dimensional ≥10mm, 5 lesions, 2/organ |
Disappearance of lesion | ≥30% decrease from baseline | Neither CR nor PD | ≥20% increase from the nadir | At least 4 weeks | Incorporated to the sum of measurement |
| itRECIST, 2020 Uni-dimensional ≥10mm, 10 lesions (5 injected, 5 not injected) |
Disappearance of lesion | ≥30% decrease from last exam for injected lesion, ≥30% decrease baseline for not injected lesion | Neither CR nor PD | ≥20% increase from the nadir (≥5mm) | 4-12 weeks | iUPD |
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; iUPD, unconfirmed progressive disease; RECIST 1.1; Response Evaluation Criteria in Solid Tumors version 1.1; immune-related RECIST, immune-related irRECIST, immune RECIST, imRECIST, immune-modified RECIST, itRECIST, intra-tumoral RECIST.
Figure 2: RECIST v1.1 and the various immunotherapeutic criteria
The earliest iterations from 2009 were based on the bidimensional WHO criteria or the RECIST criteria in 2013.Among these criteria, the handling of new lesions was complex since their diameters needed to be added to the original sum of diameters of the target lesions without clarity on requirements for confirmation of progression. Further, among the RECIST criteria variants, non-target lesions were only used to define complete response and did not contribute to defining progressive disease (PD), which was unlike the PD definition per RECIST v1.1 criteria (Figure 3).
| Target lesions | Non-target lesions | New lesions | Overall response |
|---|---|---|---|
| CR | CR | No | CR |
| CR | Non-CR/non-PD | No | PR |
| CR | Not evaluated | No | PR |
| PR | Non-PD or not all evaluated | No | PR |
| SD | Non-PD | No | SD |
| Not all evaluated | No | NE | |
| PD | Any | Yes or No | PD |
| Any | PD | Yes or No | PD |
| Any | Any | Yes | PD |
CR= complete response, PR= partial response, SD= stable disease, PD= progressive disease and NE= inevaluable.
| % change in sum of the diametersa | Non-target lesion response assessment | Overall Immune-modified RECIST Timepoint Response |
|---|---|---|
| -100% from baselineb | CR | CR |
| -100% from baselineb | Non-CR or not all evaluated | PR |
| <30% from baseline | Any | PR |
| >-30% to <+20% | Any | SD |
| Not all evaluated | Any | NE |
| ≥20% from nadir SLD | Any | PD |
CR= complete response; NE= not evaluable; PD= progressive disease; PR= partial response; RECIST= Response Evaluation Criteria in Solid Tumors; SD= stable disease; SLD= sum of the longest diameter.
a Percent change in the sum of the diameters (including measurable new lesions when present)
Figure 3: Time point assessments when both target and non-target disease are present
A. RECIST v1.1 Criteria (doi:10.1016/j.ejca.2008.10.026)
B. iRECIST Criteria (doi: 10.1200/JCO.2017.75.1644)
These discrepancies led to various modifications of irRC, irRECIST and imRECIST in clinical trial protocols, and subsequent inconsistencies and comparability issues across studies. Even validation with historical data was challenging as the post-progression scans were often unavailable. Lastly, the question remained whether what was developed for melanoma was applicable to other tumour types.
To address these issues, immune RECIST (iRECIST) was created for harmonised data collection and standardised response evaluation. It is most closely aligned to RECIST v1.1 criteria and provides a clear definition of what data are required for confirmation of PD and future successful validation for use in trial and routine settings of clinical care. In addition, iRECIST incorporates questions on clinical stability of the patient to support continuation decision on therapy, thereby providing for a true assessment of psuedoprogression seen with immunotherapeutic agents and the promise to systematically document therapy outcomes.
Further, itRECIST (intratumoral RECIST criteria) assesses the typical and atypical response to intratumoural therapies as the treatment evolves by monitoring the overall response (non-injected lesions) and local response (injected lesions). This differs from RECIST and iRECIST as they only assess systemic therapy responses.
Creating accurate databases
ASCO-Trial Reporting in immuno-oncology groups has recently recommended reporting responses according to both the conventional RECIST (primary) and iRECIST (exploratory) criteria in parallel, including spider plots to report kinetics of response and to consider integrative endpoints. [7]
Several clinical trials with immune therapeutics rely on use of both RECIST and immunotherapy specific criteria as part of either co-primary or co-secondary endpoints to enable accurate documentation of progression with immunotherapies and to support treatment dis/continuation decisions during the trial. The challenges of simultaneously using RECIST and iRECIST criteria include:
- Incorporating data entry features that will support simultaneous assessments
- Transitioning among the differing standards and into the end of the study
- Capturing treatment continuation decisions made by the investigator
- Reconciling the differing time point responses between RECIST criteria and its variants
- Protocol defining how missing confirmatory assessments will be handled
ICON specialises in creation of Case Report Forms for these response evaluation criteria to support determination of objective response rates while adhering to the highest standards of data collection per defined guidelines. Driven by a commitment to ensuring accurate records and data integrity, the team of medical experts, system designers, data managers and statisticians work together to create the case report forms that go through user acceptance testing (UAT) with several rounds of dummy data validation cycles to ensure ease of data entry and minimise deviations.
ICON’s robust data collection and data evaluation processes with sufficient baseline and follow up information will allow for future validation of response criteria and support determination of durability of response related endpoints that we might see in the future.
_____________
[1] E. Borcoman, Y. Kanjanapan, S. Champiat, S. Kato, V. Servois, R. Kurzrock, S. Goel, P. Bedard, C. Le Tourneau. Novel patterns of response under immunotherapy. Annals of Oncology, 2019; 30(3): 385-396. ISSN 0923-7534. https://doi.org/10.1093/annonc/mdz003
[2] Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical Cancer Research. 2009; 15(23): 7412-20. https://doi.org/10.1158/1078-0432.CCR-09-1624
[3] Elvire Pons-Tostivint, et al. Comparative Analysis of Durable Responses on Immune Checkpoint Inhibitors Versus Other Systemic Therapies: A Pooled Analysis of Phase III Trials. JCO Precision Oncology. 2019; 3: 1-10. DOI:10.1200/PO.18.00114
[4] Costa, Larissa B, et al. Reassessing Patterns of Response to Immunotherapy with PET: From Morphology to Metabolism. RadioGraphics, 2021; 41(1): 120-143. https://doi.org/10.1148/rg.2021200093
[5] Guan Y, Feng D, Yin B, Li K, Wang J. Immune-related dissociated response as a specific atypical response pattern in solid tumors with immune checkpoint blockade. Therapeutic Advances in Medical Oncology. 2022; 14. doi:10.1177/17588359221096877
[6] Berz, Antonia M., et al. Tumor response assessment on imaging following immunotherapy. Frontiers in Oncology, 2022, ISSN 2234-943X. https://doi.org/10.3389/fonc.2022.982983
[7] Bergamino Sirvén M, Pernas S, Cheang MCU. Lights and Shadows in Immuno-Oncology Drug Development. Cancers (Basel). 2021; 13(4): 691. doi:10.3390/cancers13040691



