SOP Management
In FDA-regulated industries, such as pharmaceutical and life sciences, standard operating procedures (SOPs) must be managed with strict change control processes to assure compliance with current GxP quality guidelines and regulations.
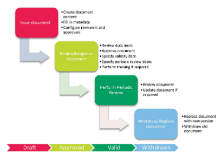
SOPs must be well structured, current, reviewed and approved in a defined workflow, and changed with traceability and documentation. Training of changing work practices, and introduction training for employees not yet trained, is a vital part of SOP management.
SOP Management and SOP Training is a part of the fully integrated Platina QMS solution and allows complete control of existing SOPs as well as initiated and ongoing SOP training programs. The solution is designed to streamline the handling of a SOP through its entire life cycle by controlling and ease creation, storage, retrieval, signing and distribution of SOPs in an electronic format.
SOP management
Platina QMS SOP Management integrates documents and SOP management into the organizations processes. The solution includes features for version control, revision control, indexing, publishing, distribution and screening. Indexing of SOPs including metadata means that the solution has powerful search tools. Publication and distribution functions are used for access and dissemination of documents, while the security checkpoints control which users have access to each specific SOP.
SOP training management
Each training process has pre-defined steps and conditions for implementation and potential trainers. It clarifies who should be trained, who trains, during which time training shall take place, etc. Moreover, training materials are stored and made available to participants. All results and certificates are recorded on each users profile or other relevant places in the system, for instance together with the current SOP so that one can easily overview trained employees for a particular SOP.