FREEZEmarker®
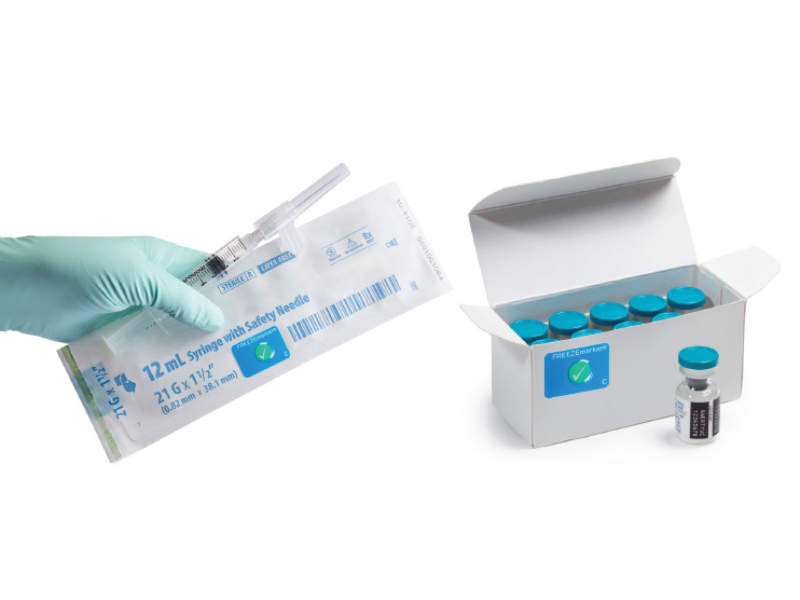
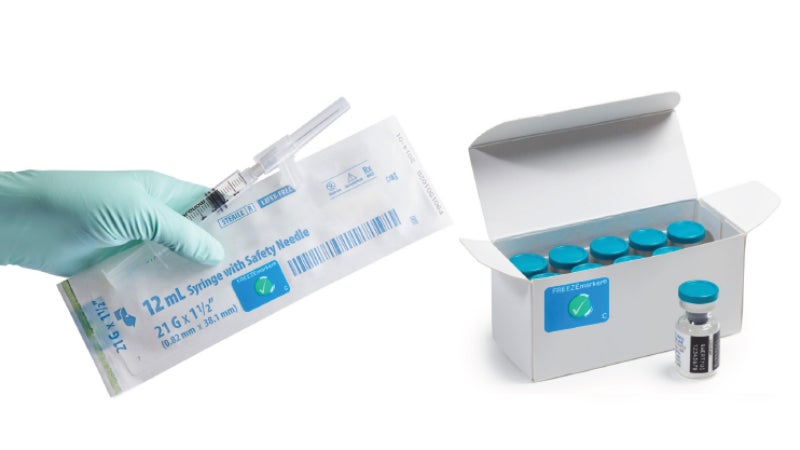
FREEZEmarker® is a single-use indicator that irreversibly changes colour from green to white to signal that a freeze event has occurred when transporting pharmaceutical goods.
A FREEZEmarker can be affixed to an individual unit to monitor the product from manufacture to point-of-care (POC), or later in the cold chain to monitor the product for a portion of its distribution. FREEZEmarker indicators are available with response temperatures of 0°C, -1°C, and -6°C.
Many medical products can be damaged and lose effectiveness if they are frozen. This is particularly true of products that require refrigerated storage. If there is a freeze event followed by a thaw, the user would have no way of knowing the freeze event occurred. Therefore, the FREEZEmarker helps identify and prevent the administration of products that have experienced a freeze event.
Key features of FREEZEmarker are:
- Irreversible
- Single use
- Easy-to-understand
- Easy-to-read
- Device activation not required
- Superior monitoring reliability
- Nontoxic materials used in product
Benefits
- Avoid administration of freeze exposed medications to patients
- Extend quality control (QC) and risk management from production to patient
- Strengthen conformance to International Code on Harmonization (ICH) Q9 and Q10
- Reduce unnecessary waste and limit the destruction of products incorrectly suspected of freeze abuse
- Support call center efforts provide definitive information about potentially freeze-damaged products to healthcare professionals and patients
- Environmentally friendly
FREEZEmarker performance and use
- Response temperatures: 0°C (±1ºC) -1°C (±1ºC) -6°C (±2ºC)
- Response time: Within 30 minutes
- Storage temperature: Refrigerated temperature (2°C to 8°C)
- Shelf-life: One and a half years to four years, determined by category
- Usage: Single use
- Temperature monitoring: Continual
- Device Size: 20mm x 29mm
- Additional features: Adhesive backing available
Quality management system
- Temptime’s quality management system is consistent with Food and Drug Administration (FDA) quality system requirement (QSR) 21 CFR 820 good manufacturing practices (GMP) for medical devices
- ISO 9001:2008
- ISO 13485:2003










