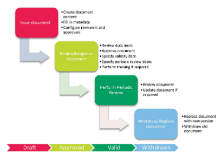
Deviation Management
It is important to manage any deviations in expected standards in the development, manufacturing and distribution processes of pharmaceutical products.
Deviations are measured differences between observed value and expected or normal value for a process or product condition, or a departure from a documented standard or procedure.
A deviation may occur during sampling and testing, raw materials- and finished product acceptance and manufacturing. Deviations may also be triggered by customer complaints or comments when the customer company’s standards do not meet critical attributes as delivered per certificate.
For compliance to GMP and the sake of continuous improvement, any deviation from established procedures needs to be documented. FDA § 211.192 requires a thorough investigation of any deviation, including documentation of conclusions and follow-up. The Quality Management System should ensure that deviations from established procedures are identified and recorded. Incidents that could affect the quality or the reliability of records or tests should be investigated and resolved.
A proactive approach to continuous quality improvement
Deviation Management is a part of Platina QMS providing efficient support for controlling deviation incidents, implementing corrective measures, helping avoid their recurrence, and for taking a proactive approach to continuous quality improvement. The system delivers deviation reports and remedial measures reports at any time, irrespective of where in the organization the deviation has occurred.
Deviation Management deals with different types of deviations such as standard (common) deviations and laboratory out-of-spec deviations. The system provides pre-defined reports for initiating any possible deviation investigation. The entire deviation process is supported by the system, from initiation and investigation, to review, approval and closure in compliance with CFR 21 Part 11.