CAPA Management

To ensure quality and efficiency when developing and operating processes, it is vital to have an optimised corrective and preventive actions (CAPA) management system.
Failure to establish and maintain procedures for implementing corrective and preventive action is an appalling yet common warning from the FDA to companies. It is vital that your CAPA system are able to minimise risk and prevent problems; otherwise it will pay during a regulatory inspection if not before.
The question you should be confident in answering is: What the quality status of your CAPA system? Does it measure up to FDA requirements? Does it improve and streamline operating processes and change management?
CAPA Management with Platina QMS
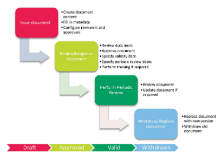
Electronic CAPA management is a part of Platina QMS solution allowing flexible management of activities related to quality and change management. From initiation to validation and acceptance our Platina QMS software solution enables excellent documentation of all steps including; investigation, testing, review, approval and closure cycles.
Platina QMS creates a complete transparency in the quality work. It provides managers real-time visibility into the critical processes. Furthermore, it allows for all employees to actively participate in the quality work through easy-to-use and pre-defined forms that initiates a CAPA process. The integrated QMS system also provides seamless traceability to related processes, e.g. Change Control and Training.