The US Food and Drug Administration (FDA) has granted approval for Ligand Pharmaceuticals’ Zelsuvmi (berdazimer topical gel, 10.3%) for molluscum contagiosum.
The approval allows the drug to be used in adults and children aged over one.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
A nitric oxide-releasing agent, Zelsuvmi is a topical medication known for its antiviral properties.
It will be commercially available in the US in the second half of this year.
Zelsuvmi is the first and only prescription drug for molluscum contagiosum that can be used in home settings.
Its efficacy was shown in the B-SIMPLE 4 and B-SIMPLE 2 Phase III clinical trials, in which it demonstrated its ability to decrease lesion counts with once-daily application and was well tolerated.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe B-SIMPLE Phase III programme enrolled a total of 1,598 subjects.
Reactions at the application site were the most commonly reported adverse reactions in the trials.
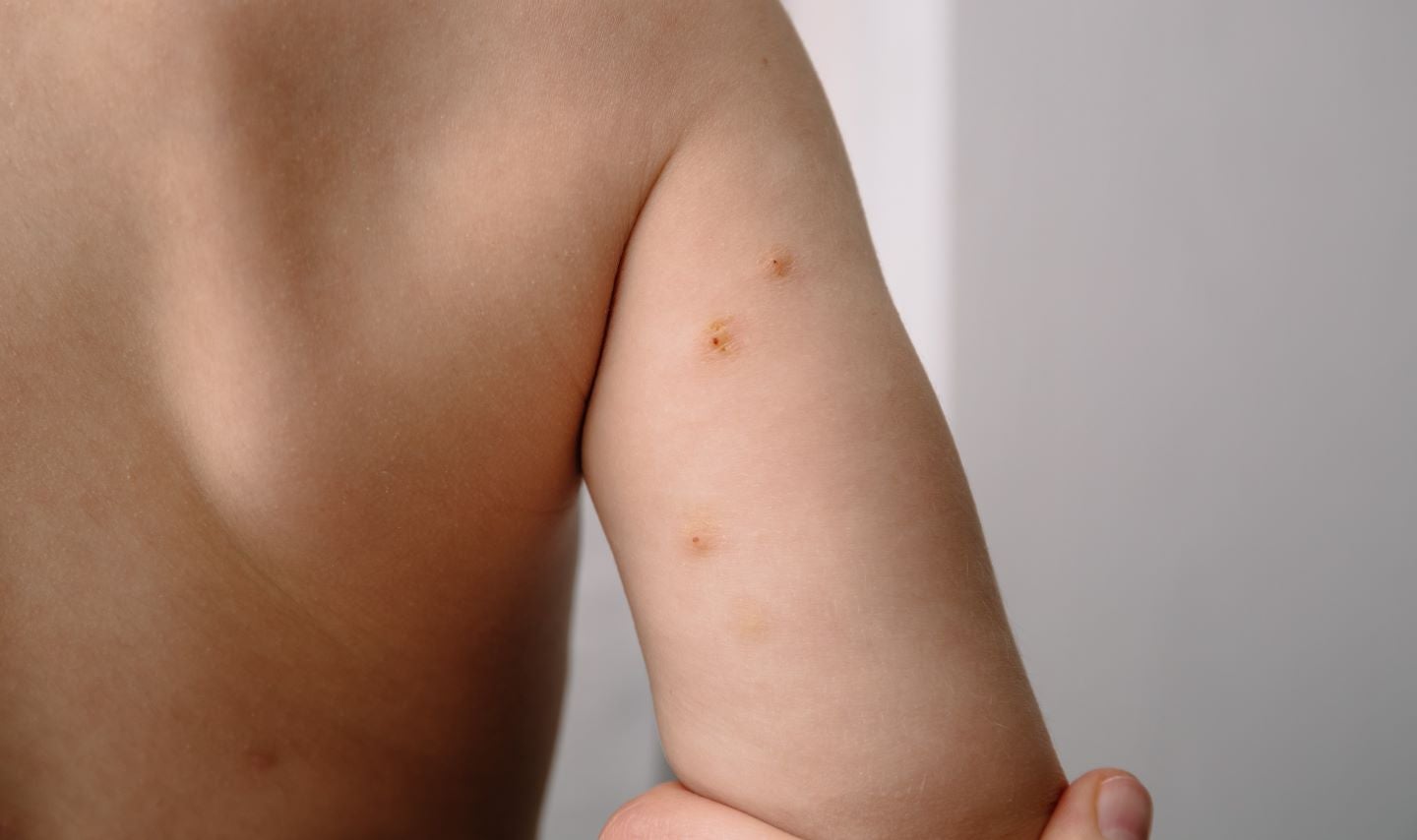
Molluscum contagiosum is a contagious viral skin ailment, presenting as lesions with an umbilicated viral core at their centre.
Treating these lesions is essential to prevent the spread of the infection.
Ligand CEO Todd Davis stated: “We are proud of the team’s accomplishment, having completed the world’s largest clinical programme in molluscum to bring this first-in-class topical medication to FDA approval.
“Paediatricians, dermatologists and caregivers have long sought a convenient approach to treat this highly contagious skin infection.
“With Zelsuvmi, patients now have an at-home treatment option available.”
In March 2022, Ligand signed a definitive merger deal to combine the business with the special purpose acquisition company Vista Public Acquisition Corp II.
Ligand then spun off its antibody discovery business, OmniAb, which became a publicly traded company.




