PRONAV Clinical
The Early Phase Clinical Supply Specialist
PRONAV Clinical provides a range of support services to reduce friction in the clinical supply process.
Subscribed
You have successfully submitted your enquiry. Someone from our company will respond ASAP
About Us

Time is critical in biotech and pharmaceutical development. The patients in your trials need their treatments delivered on time and without deviation. You need to know they’ll get it as expected and nothing disrupts your timelines like supply chain issues exacerbated by low-priority service.
At PRONAV Clinical, things work a little differently. We exist specifically to reduce and remove key pain points for emerging pharma and biotech innovators just like you. More than a service provider, working with PRONAV Clinical means gaining a partner: attentive, engaged, and enthusiastic about your success.
We deliver a seamless client experience for emerging/early-phase supply needs. How do we do it?
Attention
You and your patients are our priority—you’ll never feel like you’re at the back of the line.
- Direct liaison with leadership ensures clear understanding of your needs and challenges right from the outset.
- Engaged and knowledgeable personnel, ensure you get the correct solutions every time.
- PRONAV Clinical is focused on swift and proactive, problem resolution
Speed
Nimble and layer-free on purpose—PRONAV Clinical has the ability to deliver unparalleled service along with faster and better solutions. This agility has been a key factor in our successful support of trials with complex and dynamic factors such as: critical time frames (as in pancreatic cancers), multi-variable dosing rates (as in achondroplasia), and scheduling uncertainty (as in newborn/premature bronchopulmonary dysplasia)
Precision
Worried about cold chain management and supply issues? Worry no more. With a fluent understanding of market-specific regulations, including local packaging and labelling requirements, supported by small and large molecule Qualified Person (QP) expertise, our attention to detail and carefully qualified couriers means your cold chain investigational medicinal product (IMP) arrives safely at the site—on time, compliant, and in pristine condition.
Delivering to clinical trials sites across the globe, PRONAV Clinical has consistently maintained a 99.3% on-time delivery rate.
Solutions-driven
Inevitably, challenges arise from time to time in any clinical trial programme:
- Site initiation visits may be rescheduled
- Regulatory approvals or patient enrolment can be delayed
- Relabelling may be required to accommodate expiry date extension
- Products may need to be redirected from one geographic location to another, along with a change in language
Whatever the challenge, PRONAV Clinical is there to help. We are recognised by our clients for leaving no stone unturned in our efforts to find pragmatic solutions and ensuring that patients receive their treatment on time and that milestones are met.
Communication
PRONAV Clinical believes that timely and fully transparent two-way communication is key to the success of any project.
Using PROMAP, our proprietary Cloud-based data exchange and project management platform, our clients and other stakeholders have full visibility of all activities and associated documentation across the full supply chain. From a single online portal our project teams work in close collaboration with their client counterparts to develop the project program, identifying in detail the tasks required, the corresponding time frames, and associated documentation needs.
All aspects of the manufacturing and testing supply chain are addressed, including for example: master artworks and batch records; executed batch files; temperature-controlled storage and distribution records; regulatory and clinical trial approvals; production specification files, including relevant sections of INDs/IMPDs; and manufacturing, labelling and packaging schedules.
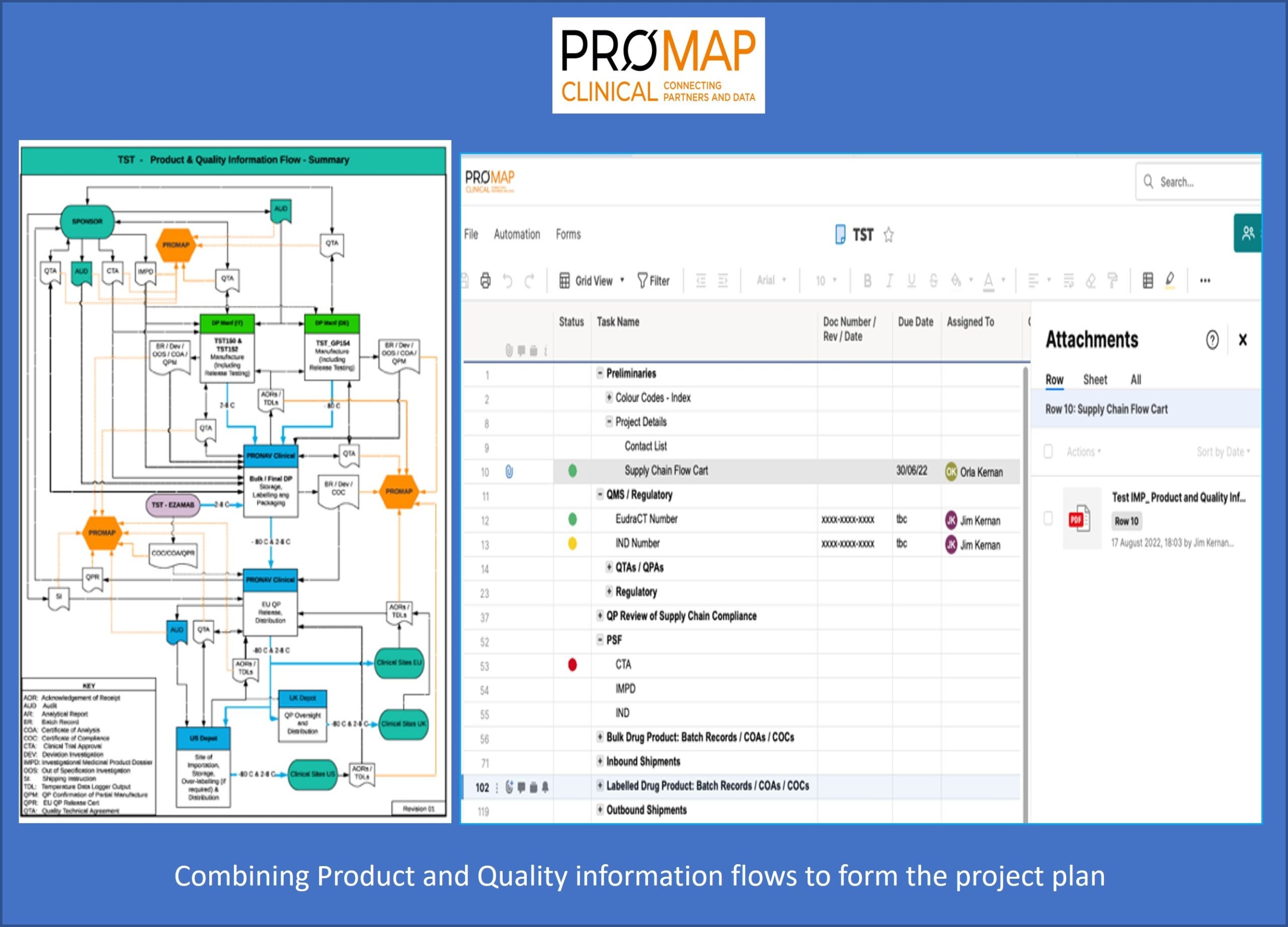
The platform is fully 21 CFR Part 11 and EU Annex 11 compliant and permits daily progress tracking, as well as direct sharing of documentation and other communication between all team members in real time. Ultimately, the single source of information embodied in PROMAP allows our Qualified Persons (QPs) to perform very efficient batch documentation reviews followed by rapid batch certification and release.
Services
PRONAV Clinical’s range of services includes:
- EU Site of Importation
- Qualified Person (QP) batch certification and release for sale
- Secondary packing of solid and liquid dosage forms
- Labelling and packaging [ambient], [2-8°C] and [-80°C]
- Blinding
- Controlled temperature storage and distribution: [-80°C] [-20°C] [2 – 8°C] [15 – 25°C]
- Inventory management and depot services
- Logistics and distribution
- Comparator sourcing
- Ancillary medicines and medical device sourcing
- Kit assembly
- Auditing of supply chain partners and QP certification
- Project management and consulting
Reputation
PRONAV Clinical has built a reputation for transparent communication and a steadfast commitment to quality. It has earned us the trust of organisations worldwide—reflected in our long-time partners and repeat clients who rely on us for seamless supply solutions.
Plan and Navigate
Supply to clinical sites located across the globe from North, Central and South America, to the European Union, the UK and the Far East, requires that PRONAV Clinical’s team has a very clear understanding of its client needs from the outset. Detailed planning and careful navigation is essential to success.
With this in mind, every project begins with a comprehensive review of relevant product and quality information flows, from drug substance manufacture, to bulk drug product formulation and filling, to IMP labelling and packaging, to QP release and, finally, to distribution. The individual tasks and data generated at each one of these steps is captured in a project-specific PROMAP. The high visibility and accessibility provided by the platform ensures that potential challenges are identified and resolved early on.
Available online across time zones, and with direct communication capability between team members from within the platform, PROMAP greatly enhances the efficiency with which issues are identified and resolved, and ultimately, the speed with which IMPs can be manufactured, tested, released, and shipped to sites across the globe.
Reach out today—let’s talk about where you’re going next and how we can meet you there.

White Papers
Contact Details
Website
Email Address
Address
Carraroe,
Ireland,
F91 D439
