Using a Developmental Chamber During Ethylene Oxide Validation
The authors of this white paper guide readers through the use of a developmental chamber for Process Definition in adherence with ISO 11135:2014.

Sterigenics is the global leader in comprehensive sterilization solutions and Expert Advisory Services providing mission-critical and government-mandated sterilization services for more than 90 years across gamma, ethylene oxide (EO), electronic beam (E-Beam) and X-Ray technologies.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Sterigenics is a global leader in comprehensive sterilization solutions and expert advisory services, providing mission-critical and government-mandated sterilization services for more than 90 years across gamma, ethylene oxide (EO), electronic beam (E-Beam) and X-Ray technologies.
With applications ranging from pharmaceutical drug products to medical device sterilization, these technologies are suitable for sterilization, microbial reduction and material modification.
Sterigenics is a business unit of Sotera Health along with Nordion® and Nelson Labs®. Together, these companies are world-leading, fully integrated protectors of global health, touching the lives of more than 180 million people around the world each year.
Sterigenics aims to develop and maintain the most effective sterilization processes for its pharmaceutical clients’ products, bringing them to market in the fastest, most efficient and safest way possible.
Sterigenics also offers sterilization across a number of other industries, including, medical device, food, commercial products, packaging and advanced radiation processing.
Sterigenics’ radiation-based gamma sterilization solution offers a wide range of polymer compatibility with some limitations due to oxidation effects.
Products can be shipped to the final user as soon as they exit the gamma irradiation facility.
Applications of gamma sterilization include:
EO offers a wide range of material compatibility, except for moisture and temperature-sensitive materials.
For manufacturers whose products are particularly radiation sensitive, EO can be an effective sterilization modality.
Applications of EO sterilisation include:
Nitrogen Dioxide (NO2) based sterilization offers ultra-low temperatures, minimal pressure requirements, no cytotoxic residuals, and fast cycle times.
Applications of NO2–based sterilization includes external sterilization of pre-filled syringes, vials or cartridges where the goal is that NO2 does not reach the pharmaceutical product.
Sterigenics is a leading global provider of comprehensive sterilization solutions and expert consulting services, offering:

The authors of this white paper guide readers through the use of a developmental chamber for Process Definition in adherence with ISO 11135:2014.

Our goal is to develop and maintain the most effective sterilisation processes for your products, bringing them to market in the fastest, most efficient and safest way possible.

Sterigenics is pleased to announce that it will be making its return to BIOMEDevice in San Jose, CA on 4-5 December.

We are delighted to be once again exhibiting at CPhI WW, taking place on 5-7 November at Frankfurt Messe in Frankfurt, Germany to take part in its 30th birthday celebrations.

Sterigenics has announced that it will be attending CPhI North America.

Sterigenics has announced that it will be attending Pharmapack, Paris.

Sterigenics has announced that it is building a new facility in Markham Vale North in Chesterfield/Derbyshire, UK.

Sterigenics has announced that it is building a new facility in Markham Vale North in Chesterfield/Derbyshire, UK.

Sterigenics International has announced that it has acquired Toxikon Europe, the European division of Toxikon Corporation.

Noxilizer and Sterigenics International have announced the signing of a global agreement that will make Sterigenics the exclusive worldwide provider of nitrogen dioxide (NO2) contract sterilization services, as well as feasibility and research studies, to the pharmaceutical, biotech and medical device manufacturing industries.

Sterigenics will be attending Interphex 2015 from 21-23 April at Javits Center in New York.

Sterigenics International has announced its acquisition of Gammarad, Italy's leading gamma sterilisation provider.

Niki Fidopiastis, SteriPro's consulting director, will serve as a trainer at the Radiation Sterilization for Medical Devices Training Programme from October 7-10 2014 in Alexandria, VA.

Sterigenics International has announced it has completed the $826m acquisition of Nordion Inc.

Sterigenics International has announced that it received ISO / IEC 17025:2005 food and chemistry accreditation in Mexico.

Sterigenics has announced it will invest more than $10m to expand operations in Atlanta, Dallas and Belgium.

Sterigenics has entered into a definitive agreement to acquire Nordion Inc.

Sterigenics International has announced it has closed the acquisition of Food Technology Service Inc.
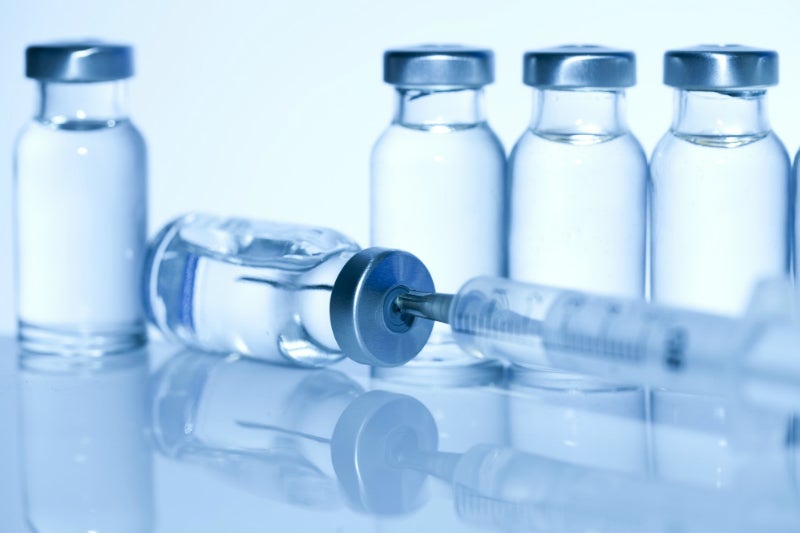
Ethylene Oxide (EO) offers a wide range of material compatibility except for moisture- and temperature-sensitive materials.

Nitrogen Dioxide (NO2)-based sterilization offers ultra-low temperature, minimal pressure requirements, no cytotoxic residuals, and fast cycle times.

Gamma offers a wide range of polymer compatibility with some limitations due to oxidation effects.