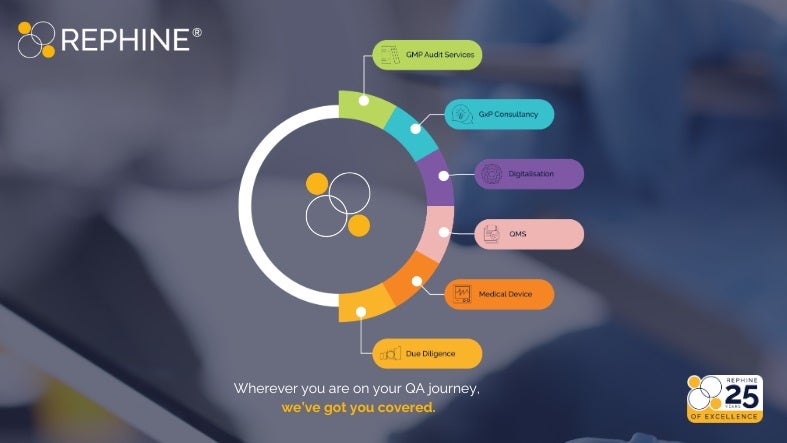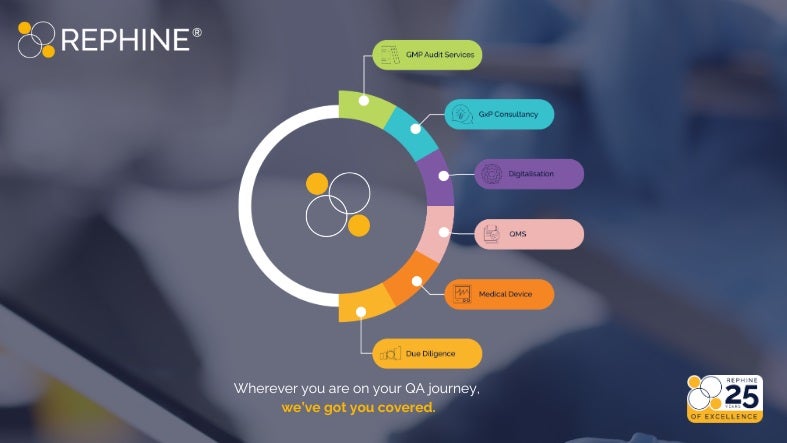
Rephine is a globally recognised leader in product quality assurance and compliance for the highly regulated life sciences sector, including pharmaceutical, biotechnology, and medical device manufacturing supply chains.
Specialising in good practice (GxP) readiness, digital transformation of manufacturing controls, and quality management system advisory services, Rephine ensures compliance and operational excellence across the entire product development lifecycle—from raw materials and device components to patient delivery.
Offering tailored solutions for the medical device market, comprehensive auditing services, and cutting-edge robotic capabilities, Rephine has earned its reputation as the gold standard in pharmaceutical quality assurance.
Pharmaceutical quality compliance auditing and reports
Rephine’s auditing services are renowned for their thoroughness, consistency, and reliability. We offer on-demand audits, as well as revalidated off-the-shelf audit reports, to help clients assess and improve their quality compliance status.
Our auditors are highly experienced professionals with deep knowledge of global regulatory requirements. They provide actionable insights and recommendations to address deficiencies and enhance quality systems.
By partnering with Rephine, companies can ensure that their operations meet the highest standards of quality and regulatory compliance.
Comprehensive GxP readiness services
Rephine’s GxP readiness services are designed to help life sciences companies meet stringent regulatory requirements. Whether good manufacturing practice (GMP), good laboratory practice (GLP), or good clinical practice (GCP), Rephine ensures that manufacturers are fully compliant with global standards.
Our team of experts works closely with clients to identify gaps, implement corrective actions, and prepare for regulatory inspections. By leveraging our deep industry knowledge and proven methodologies, we help businesses mitigate risks, avoid costly delays, and maintain uninterrupted operations.
Digital transformation of manufacturing controls
In an era where technology drives efficiency, Rephine is at the forefront of digital transformation in manufacturing controls. Our services include the integration of advanced robotic capabilities, automation, and data analytics to optimise production processes.
By digitising quality control systems, we enable manufacturers to achieve greater accuracy, consistency, and traceability. This not only enhances operational efficiency, but also ensures compliance with evolving regulatory demands.
Rephine’s innovative approach empowers clients to stay ahead in a competitive market while maintaining the highest standards of product quality.
Quality management system (QMS) advisory services
A robust quality management system is the backbone of any successful life sciences organisation. Rephine offers tailored QMS advisory services to help companies establish, implement, and maintain effective quality systems.
Our experts provide guidance on document control, risk management, change control, and continuous improvement initiatives. By aligning QMS processes with regulatory requirements and industry best practices, we help clients achieve operational excellence and build a culture of quality throughout their organisation.
Tailored solutions for the medical device market
The medical device industry faces unique challenges, from stringent regulatory requirements to rapid technological advancements. Rephine’s specialised services address these challenges head-on, offering customised solutions for device manufacturers.
Our expertise spans the entire product lifecycle, from design and development to post-market surveillance. We help clients navigate complex regulatory landscapes, ensure compliance with standards such as ISO 13485, and implement risk management strategies.
With Rephine’s support, medical device companies can deliver safe, effective, and high-quality products to market.
Commitment to life science quality assurance
Rephine’s commitment to excellence is reflected in every aspect of our work. Our honed processes, bespoke service design, and appropriate use of technology set us apart as the gold standard in life science quality assurance.
We understand the complexities of the life sciences industry and are dedicated to helping our clients achieve their goals. With a global presence and a team of seasoned experts, Rephine is the trusted partner for companies seeking to ensure product quality, regulatory compliance, and operational efficiency.
About Rephine
Founded with a vision to redefine quality assurance in the life sciences industry, Rephine has grown into a globally respected organisation. Over the years, we have built a reputation for delivering exceptional results through our innovative solutions, unwavering commitment to quality, and customer-centric approach.
Today, Rephine continues to lead the way in product quality assurance, helping life sciences companies ensure their products and services are of the highest quality.