Implementing Pharmaceutical Serialisation Across a Diverse Life Science Manufacturing Environment
US and European healthcare industries are changing rapidly, with organisations facing the challenge of adopting new regulations for pharmaceutical serialisation.
With deadlines for implementing track and trace processes firmly on the horizon, manufacturers should be considering the best approach to implement new pharmaceutical serialisation solutions in time.
In a piece for Manufacturing Chemist and serialisation director at Zenith Technologies Carlos Machado, he explores the challenges linked to pharmaceutical serialisation strategies and deployment, as well as the benefits that can be gained from taking a proactive approach to implementation.
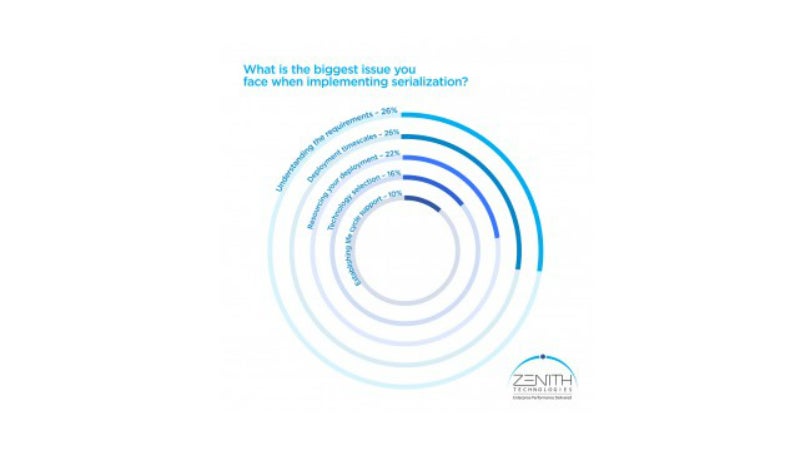
In a recent poll survey of 100 pharmaceutical manufacturing professionals, Zenith asked what the biggest issues were that companies face when implementing serialisation.
Statistics show that global counterfeit drug sales have reached $75 billion annually and the World Health Organisation (WHO) reports that 16% contain the wrong ingredients. This indicates the seriousness of the falsified medicines crisis and it is statistics like these that have been a key driver for change within the industry.
With pharmaceutical serialisation compliance requirements having already been implemented in parts of the globe, including China, India, South Korea and Saudi Arabia, the US and the EU are quick to follow, with deadlines in 2017 and 2019 respectively.
Carlos explains the steps pharmaceutical manufacturers should take to remain compliant, including understanding the scope and impact of the project and having a defined project plan in place.