VIALEX™ - Premium Technology for Surface Durability
VIALEX™ completely eliminates the need for ammonium sulfate treatment due to the limited shift in pH resulting from a significant reduction of borate deposits on the glass surface.

NIPRO PharmaPackaging is a leading manufacturer of advanced primary packaging solutions and medical devices to the pharmaceutical industry
You have successfully submitted your enquiry. Someone from our company will respond ASAP

NIPRO PharmaPackaging specialises in developing and manufacturing advanced primary packaging solutions and strives to be the preferred and trusted partner for pharmaceutical companies worldwide in the safe delivery of parenteral medicines to patients.
NIPRO PharmaPackaging is part of NIPRO Corporation, Japan, established in 1954. As a leading global healthcare company with over 39.000+ employees worldwide, NIPRO serves the Pharmaceutical, Medical Device, and Pharmaceutical Packaging industries.
NIPRO PharmaPackaging (NIPRO) is a vertically integrated partner to the pharmaceutical industry, managing the full process from raw materials to final delivery.
By producing its own high-quality glass tubing and manufacturing billions of containers annually, NIPRO ensures consistent quality and helps customers select the optimal packaging for their drug products.
NIPRO offers an extensive range of high-quality glass primary packaging solutions, including ampoules, dental and pen cartridges, D2F™ Syringes, and D2F™ vials¹.
As a provider of complete injection systems, we also supply all essential components (closures, stoppers, plunger rods, caps, etc.) and accessories (backstops, safety systems, etc.).
Recognising the widespread use of combination products in the industry, we collaborate with device manufacturers to ensure the seamless integration of cartridges and pre-fillable syringes into injection devices, providing necessary compatibility statements.
Additionally, NIPRO offers a selective range of medical devices, such as Curacase needles and D2Mix devices, designed to facilitate safer and more efficient reconstitution and administration processes.
NIPRO operates one of the largest manufacturing networks in the glass primary packaging industry. We currently have 13 state-of-the-art production sites across Asia, Europe, and America.
Our global presence allows business partners to source packaging solutions worldwide with local production. This results in faster supply times, risk mitigation through multi-sourcing, and enhanced sustainability due to shorter supply chains.
To produce our medical device range, we collaborate with NIPRO’s medical division, a global market leader in healthcare products known for meticulous production processes and premium quality.
All our plants are highly automated, equipped with advanced machinery, and utilise sophisticated dimensional and cosmetic in-line inspection systems to ensure your products are manufactured precisely to agreed specifications.
Developing a new packaging solution that meets the specific requirements of a drug product and integrating it into the fill-finish process is a complicated journey. NIPRO’s highly trained, customer-focused service is here to support you every step of the way.
Our dedicated team includes experts in sales and customer service, regulatory and quality affairs, as well as technical support. In addition, our in-house laboratory services (ISO 17025-certified) provide analytical testing and studies to help optimise the way packaging solutions perform.
From development to commercialisation, you’ll be supported throughout the entire product life cycle by a competent, cross-functional team. We ensure your inquiries are addressed quickly and efficiently, with a full commitment to your success.
When it comes to medication and patient well-being, quality is paramount. Our processes are governed by stringent quality systems, including ISO 15378, ISO 13485, ISO 45001, ISO 14001, and ISO 50001.
Our glass tubing, primary container, and medical devices comply with the applicable ISO standards, Pharmacopoeias, industry standards, and MDR. To ensure consistent high product quality across all our plants, we continuously improve our global equipment, processes, and technologies.
NIPRO is deeply committed to sustainability, aiming to significantly reduce its environmental impact while continuing to deliver high-quality products and services.
NIPRO has a clear vision and global sustainability roadmap to achieve ambitious ESG goals such as SBTi objectives, EcoVadis raking ≥ silver, ISO 50001 on all sites and a company-wide carbon neutrality by 2045.

VIALEX™ completely eliminates the need for ammonium sulfate treatment due to the limited shift in pH resulting from a significant reduction of borate deposits on the glass surface.

Nipro, with more than 60 years of experience in converting standard and customised vials, offers a wide range of high-quality vials that meet the diverse requirements of drug products.

Nipro PharmaPackaging is a long-standing and trusted partner of leading pharmaceutical companies for the development and manufacture of high-quality parenteral packaging.
Complex biological drugs and biosimilars are transforming the healthcare market by offering treatments for diseases that were hitherto seen as incurable.

Nipro's Centre of Excellence for high-quality pre-fillable glass syringes is located in Münnerstadt, Germany, in the heart of Europe. The state-of-the-art plant manufactures a wide range of D2F™ (Direct-to-Fill) glass pre-fillable syringes. Due to the many benefits glass pre-fillable syringes offer to the pharmaceutical companies and patients alike, the demand is growing continuously.

Millions of people are currently physically affected by the coronavirus (Covid-19), and the run for effective treatments and vaccines is in full swing. More than 350 drugs are under development, destined to be administered to patients by injection. More than two-thirds of these are complex, large molecule drugs, which have significant quality requirements towards their primary packaging.

The coronavirus (Covid-19) is currently the medial centre of attention, forcing other important topics like environmental protection to come second. Although Nipro PharmaPackaging is very engaged in manufacturing and delivering substantial numbers of glass vials for Covid-19 vaccines to pharmaceutical companies, previously determined environmental goals still apply.

The launch of the CURACASE™ needles further strengthens our ability to manufacture and supply complete injection systems for the pharmaceutical industry.

To further contribute to the well-being of patients and healthcare professionals alike, Nipro PharmaPackaging offers a range of safety systems compatible with Nipro pre-fillable glass syringes. We are delighted to expand our portfolio, announcing that Nipro D2F™ pre-fillable glass syringes have been tested and are fully compatible with Schreiner MediPharm’s Needle-Trap.

Our high-quality glass tubing has been the cornerstone for leading primary packaging converters, enabling the production of premium RTU syringes, RTU vials, pen cartridges, and ampoules for the pharmaceutical industry.

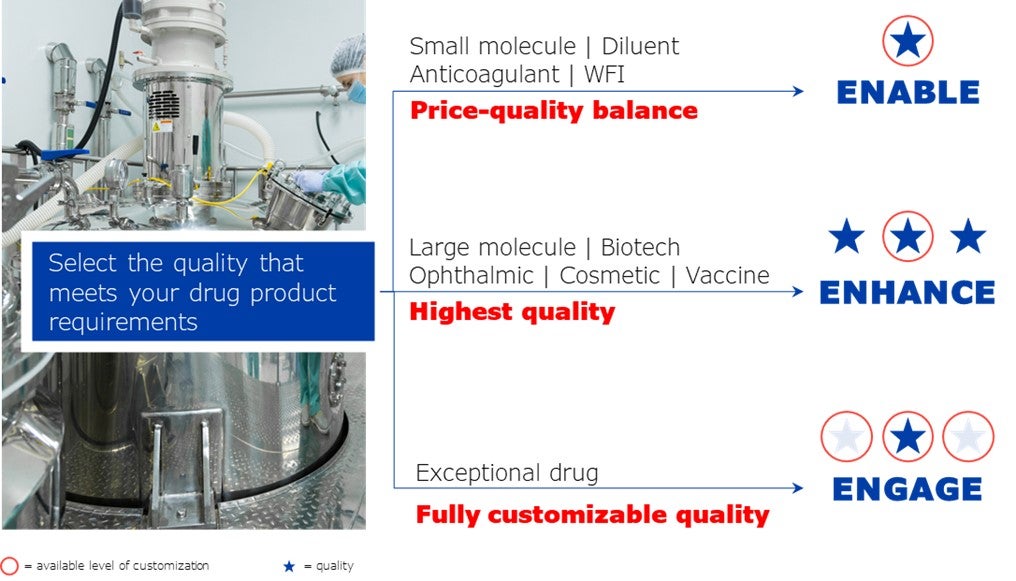
As a leading manufacturer of high-quality pre-fillable glass syringes for the pharmaceutical industry, NIPRO offers packaging solutions for different quality levels (ENABLE, ENHANCE, ENGAGE).

NIPRO, a trusted manufacturer of high-quality RTU glass vials, delivers tailored solutions for both conventional and advanced pharmaceutical formulations.

NIPRO offers high-quality dental and pen cartridges that ensure reliable fill-finish operations and are designed for precise integration into injection pens and injection devices.

Hypodermic needles are often processed on packaging lines. The unit pack design of the Curacase needles is specifically designed to support compatibility with feeders and pick-and-place systems.

NIPRO offers a wide range of high-quality ampoules that contribute to reliable fill-finish operations and provide stable break force for consistent, easy opening.