Development of a Disposable Grade-A Aseptic Fill/Finish Isolation System
While design characteristics may be identical to rigid stainless-steel isolators, flexible film aseptic isolators offer several advantages for careful consideration.

ILC Dover offers flexible powder containment technologies, eliminating problems associated with the use of traditional metal and glass systems for the pharmaceutical industry.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

ILC Dover offers flexible powder containment technologies, eliminating problems associated with the use of traditional metal and glass systems for the pharmaceutical industry.
The company applies flexible solutions to pharmaceutical potent powder containment during processing of bulk compounds and oral solid dosage manufacturing throughout the industry from North America to Europe and Asia.
As demonstrated by the Risk-Based Manufacture of Pharmaceutical Products (Risk-MaPP) principles, both current good manufacturing practices (cGMP) and industrial hygiene (IH) needs can be met by containing the process at the source. By employing flexible containment and using the logic diagrams from the Risk-MaPP process, cleaning is minimised and the operator is protected with this engineering control. As such, processes in multi-product facilities can be safely performed without the risk of cross contamination.
The DoverPac® containment system is a highly reliable storage solution for disposable products and single-use powders. Suitable for charging and offloading procedures, the system comprises a tough film liner supported by a durable fabric outer restraint.
The liners are formulated to meet client requirements, delivering high elongation performance for ruggedness and strength. This is complemented by a unique blend of anti-static additives, allowing for the safe and secure containment of pharmaceutical products.
DoverPac® has gained wide acceptance by the pharmaceutical industry for use during processing of bulk compounds. DoverPac® prevents worker exposure to highly potent compounds and can eliminate the need for protective garments and respirators.
The new EZ Biopac is a contaminant and transfer system designed for easy adjustment to different bulk weights. Achieving a precice and quick fill time, the system keeps the discharge outlet seperate from antistatic polymer material resulting in 2g or less residule in a 5kg bag. In addition, the EZ Biopac has a protective bag to help ensure the support stand and exterior are not contaminated.
ILC Dover’s flexible containment technologies allow continuous, contained processing utilizing ArmorFlex™ films developed specifically for the pharmaceutical industry. DoverPac® fabrication techniques are based on over 50 years of experience in life-critical product manufacturing.
Unlike standard flexible intermediate bulk containers that are not designed specifically for potent compound manufacturing, DoverPac® is designed to assist operators in performing operations without ever opening up the system. And, our ISO: 9001 registration assures product quality and operator safety during pharmaceutical potent powder containment operations.
Flexible containment is a validated process that uses high-quality engineered storage products with a five-year shelf-life. They pass Chilworth incendivity tests, contain antistats to prevent powder sticking to the side of the bag, and have a drug master file (DMF) filed with the FDA.
ILC’s innovative ArmorFlex containment material is compliant with US Food and Drug Administration (FDA) and EU requirements:
Flexible containment is a validated process that utilises quality engineered products designed to meet critical requirements for:
DoverPac® is one of the fastest growing containment solutions in the industry. Our worldwide clients have appreciated the ability to upgrade existing facilities for potent compound containment during processing. This ability to upgrade has allowed our clients to avoid the capital expense of new equipment and / or facility upgrades. And, the fast turn around to facility upgrading for pharmaceutical containment has meant significant decreases in facility start-up times.

While design characteristics may be identical to rigid stainless-steel isolators, flexible film aseptic isolators offer several advantages for careful consideration.

A pharmaceutical production line must be safely and efficiently managed above all else. If a product becomes contaminated or production is delayed, it can have severe ramifications for the manufacturer responsible.

In May 2019, ILC Dover opened a manufacturing facility in Ireland to produce the company’s pharmaceutical and biopharmaceutical product lines. The plant is designed to strengthen the company's single-use powder handling solutions business.
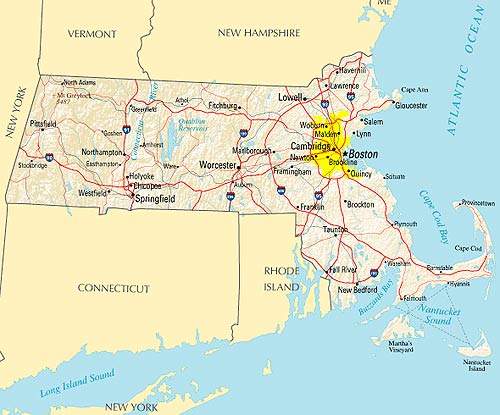
In June 2006, US-based pharmaceutical company Bristol-Myers Squibb pledged to build a large-scale multi-product biopharmaceutical manufacturing plant in Devens, Massachusetts.
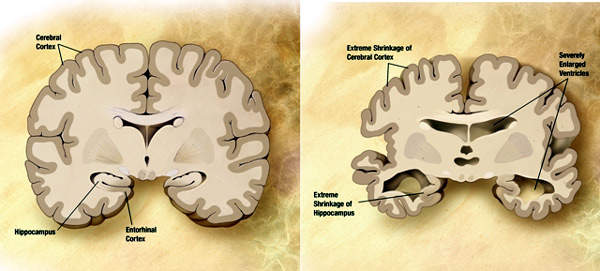
Eli Lilly made a commitment to invest approximately $400m in a biologics manufacturing facility in December 2006.

Wacker Chemie inaugurated a new production facility at its Jena site on 8 March 2010. Located approximately 90km south-w

US formulation development company CoreRx Pharmaceuticals (CoreRx), initiated a major expansion of its manufacturing sit
Canadian contract drug maker Patheon, completed the expansion of its Toronto manufacturing facility in April 2009, two m

SAFC Pharma is a multinational operation, part of the US fine chemical company Sigma-Aldrich. SAFC specialises in provid

Bristol-Myers Squibb (BMS) has been undergoing much the same rationalisation process as many large pharmaceutical compan

Puerto Rico has a growing reputation as a biotech and pharmaceutical hub ('Bio Island') and plays host to manufacturing facilities from the world's largest pharmaceutical companies.
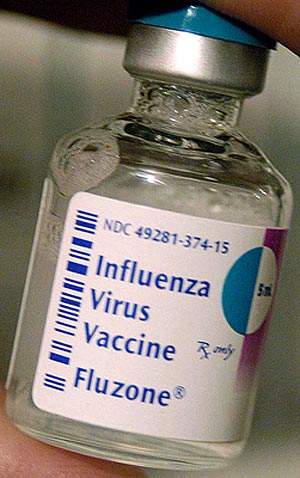
Sanofi Pasteur, the vaccines business of Sanofi-Aventis, is one of the largest seasonal influenza vaccine producers in t
Using single-use technologies to achieve continuous manufacturing has long been a goal of biomanufacturers.

Today, manufacturers are optimising for open-suite facility designs to maximise throughput and profitability.

ILC Dover develops flexible isolators (glove bags) for the pharmaceutical industry.

The DoverPac® Containment Systems are the global premier products for disposable process and powder containment systems.

Automated air controls for containment that protect people and the environment from exposure.

The CrimpLoc® System provides high containment and secure closure when used with our ArmorFlex® films.

The EZ BioPac speeds up media and buffer production with modern single-use powder handling.