Contract Immune Monitoring and Research
Establishing new bioanalytical laboratory assays requires a major investment of effort and capital. You can let CTL's team of experts handle the details for you, to save time and money.

Cellular Technology Limited (CTL) provides contract laboratory services to the pharmaceutical and biotechnology industry, specialising in cell mediated immune monitoring for research, clinical trials, and preclinical studies. Its services are also utilised by government agencies and academic institutions.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
Cellular Technology Limited (CTL) provides contract laboratory services to the pharmaceutical and biotechnology industry, specialising in cell mediated immune monitoring for research, clinical trials, and preclinical studies. Its services are also utilised by government agencies and academic institutions.
The company is well-known for its experience with the enzyme-linked immunospot (ELISPOT) assay monitoring method and is one of the leading companies in its field. CTL has established instrumentation that helped define ELISPOT research parameters for cell-mediated immunity. Other focuses include multiplex cytokine bead arrays (CBA), flow cytometry (such as phenotyping and multimer readouts), and BrdU proliferation assays.
This privately held biotechnology firm’s facilities are good laboratory practice (GLP) compliant and Clinical Laboratory Improvement Amendments (CLIA) certified, with headquarters in Shaker Heights, Ohio.
CTL has a wealth of experience with a wide range of assay types, including enzyme-linked immunosorbent assay (ELISA), multiplex cytokine bead arrays (CBA), flow cytometry for cell phenotyping, and multimer readouts, with particular expertise in ELISPOT and FluoroSpot. These have been used in both regulated and non-regulated studies.
ELISPOT assays and multiplex FluoroSpot technology can be used to measure direct ex-vivo cytotoxicity assays and per-cell antigen-specific cytokine production for applications such as T-cell functional activity studies and epitope mapping.
CTL’s novel ELISPOT and FluotoSpot assays are also used to detect and analyse Immunoglobin G (IgG) subclasses produced by antigen-specific B cells in the same assay well, with up to four antibody classes detected simultaneously. This means large-scale screening is more effective and efficient, using a much smaller amount of cell material in comparison with previous methods.
CTL has a wide variety of test systems, including human, non-human primates, pig, and mouse studies, which are all certified, optimised, and qualified.
For clinical multisite studies, CTL serves central blood processing laboratories by providing services such as logistic support for shipment of blood for maintained functionality and separating peripheral blood mononuclear cell (PBMC), plasma, or subpopulations as requested from the whole blood, cryopreserve, and cryo-storage services with on-site facilities.
CTL offers clinical, preclinical, and non-clinical contract laboratory services, compliant to Clinical Laboratory Improvement Amendments (CLIA), 42 CFR 493; and US Food and Drug Administration (FDA) regulations 21 CFR Part 58 and 11, with particular expertise in high-throughput T-cell monitoring. The company ensures quick turnaround times, offering testing, processing, and analysis of up to 1,000 samples for a significant acceleration of drug development.
From specimen processing to data acquisition, analysis, and quality control (QC), CTL has expertise in high-throughput immune monitoring. Undertaken in CTL’s Contract Laboratory facility, the company also develops and validates test methods to suit clients’ needs, while adhering to various FDA requirements. In addition, the company’s ELISPOT assays and multiplex FluoroSpot technologies are ideal for measuring specific per-cell cytokine production and direct ex-vivo cytotoxicity assays.
Further laboratory services include testing of T-cells for the simultaneous secretion of multiple cytokines, T cell functional avidity studies, epitope mapping, and B cell ELISPOT assays for determining the class or subclass of antibody-secreting cells.
As part of its extensive standardization packages for cellular assays, CTL offers serum-free media solutions and cryopreservation reagents, ELISPOT reference sample QC sets, standardized antigen (CEF peptide pools), and cryopreserved PBMC, which is characterized for HLA types and established antigen reactivity.
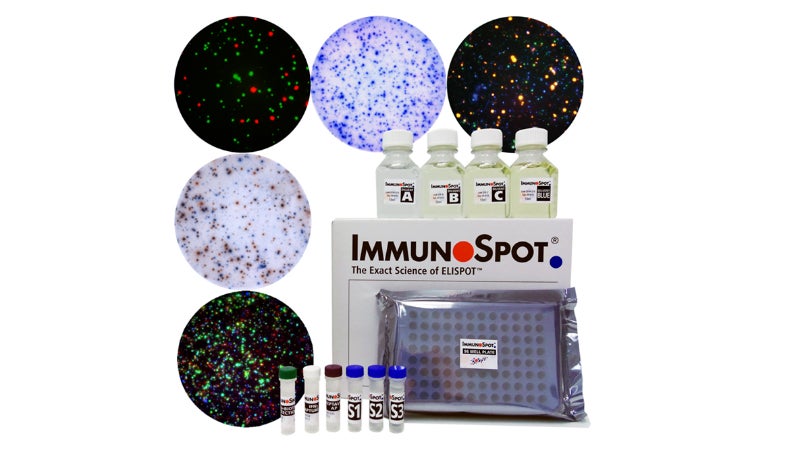
Using analysis of ELISPOT and FluoroSpot assays, CTL develops and manufactures the ImmunoSpot line, image-based analysis devices that support customer compliance to FDA 21 CFR Part 11. Able to analyse both visible and fluorescent light, the instrument uses spot-counting principles.
CTL has become one of the top worldwide manufacturers of these instruments and software. Each unit is manufactured under an ISO 9001:2015 certified quality management system.
Over the years, CTL has optimized and refined its instrumentation to serve a wide range of pharmaceutical testing requirements. The BioSpot analysis platform has a large number of applications, including yeast viability assessments, clonogenic and genotoxicity assays, viral plaque assays, and bacterial colony counting.
In addition, bioassay analysis often requires error-free visual evaluation of complex optical patterns in test wells. CTL’s ImmunoSpot analyser evaluates assays accurately, while leaving transparent audit trails.

Establishing new bioanalytical laboratory assays requires a major investment of effort and capital. You can let CTL's team of experts handle the details for you, to save time and money.

Cellular Technology Limited is an expert in ELISPOT technology, specialising in developing ELISPOT instrumentation and assays, as well as their implementation in good laboratory practice (GLP) compliant contract research.