A New DPI Device for the Generic Drugs Market
Gerresheimer has assumed responsibility for the industrialization of a dry powder inhaler for the treatment of respiratory ailments for MERXIN (United Kingdom), a company that specializes in making inhaler devices. The inhaler is produced in Pfreimd (Germany) for worldwide distribution. Beyond the technically sophisticated industrialization of the product, the main challenge of the project was to coordinate an optimal development process aimed at ensuring the shortest possible time to market at the lowest cost.
MERXIN MRX003 capsule dry powder inhaler is used for pulmonary delivery and in particular for the treatment of chronic, obstructive pulmonary diseases (COPD) and asthma. The API is aerosolized and distributed with the respiratory system to find its way deep into the lungs of the patient. The correct interplay of inhaler and formulation plays a decisive role in the success of the treatment.
The first product target for MRX003 was a generic version of an API. Because of the nature of the generic market, it was essential to achieve the shortest possible time to launch. This was achieved through close cooperation between the partners.
Decades of expertise and know-how in inhaler design and production were combined between Gerresheimer and MERXIN. Jochen Wegerer (Program Manager, Gerresheimer Regensburg GmbH, Wackersdorf) was impressed by the cooperation: “The good teamwork within the project is noteworthy. Challenges were always discussed in a goal-oriented, creative, and open manner, so that approaches to solutions could be formulated and implemented within the shortest time possible.”
The design of the manufacturing process of MRX003, had to deliver high product quality with the most stable, fully automated processes possible and to enable affordable production for the generic drugs market. “With the help of our DMF package, we were able to create, implement, and qualify the molds to be very robust,” Richard Gradl (Mold Engineer, Gerresheimer Regensburg GmbH, Wackersdorf) explains. “The stable component quality and high process capability of the molds ensure good conditions in the series production environment.”
Our risk management approach was based on procedures that were tailored to the special features of the project, from qualification and validation to long-term production security, as highlighted by Tobias Bernklau (Global Head of Quality Engineering, Gerresheimer Regensburg GmbH, Wackersdorf): “The focus is always on the user for all decisions. For critical areas and functions, we deliberately invest more effort than for less critical areas and functions.”
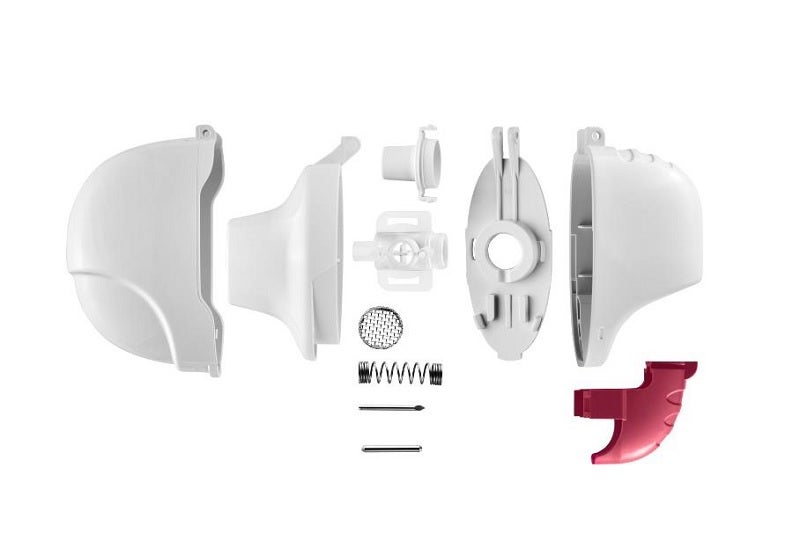
MRX003 is assembled from a total of 12 parts, meaning seven injection molding parts of ABS or MABS (base element, capsule housing, hinge plate, button, mouthpiece, filter housing, sealing cap) as well as five stainless steel bought-in components (two lancets, spring, cylinder pin, and fine filter unit). A pouch is used for the packaging of the finished product. For the BICs, Gerresheimer selected suppliers who are capable of fulfilling sophisticated quality. The injection molding parts are produced at the Gerresheimer location in Pfreimd in multi-cavity tools. The fully automated assembly and gap-free testing of each individual inhaler takes place at three round table stations.