Founded in 1947, GOLLER Systems is a contract development and manufacturing organisation (CDMO) that focuses on the development and production of medical devices and primary packaging.
Our product range includes bespoke injection-moulded plastic parts, partly assembled single components, and complex customised systems.
Customised product development for the medical sector
GOLLER Systems specialises in tailored system solutions for clients in the pharmaceutical, diagnostics and medical industries. Together with our client base, we develop innovative products, bring them to maturity and carrying out serial production.
In our in-house design and construction department, we can quickly realise product ideas with 3D computer-aided design (CAD), injection moulding simulation and rapid prototyping (also using 3D printing). As a result, we offer flexibility during the initial stages and can integrate changing requirements efficiently into the process.
In addition to new product development, we are also available for the transfer, further development and serial transfer of customer concepts, accompanying our clients at market launch or during clinical trials.
Injection moulding in cleanroom environments
As experts in the field of plastic injection moulding for medical and pharmaceutical applications, GOLLER is an ideal partner for demanding projects due to our use of state-of-the-art technology, efficient processes, and our experienced and committed team.
Our high-performance injection moulding machines have clamping forces between 500kN and 1,500kN.
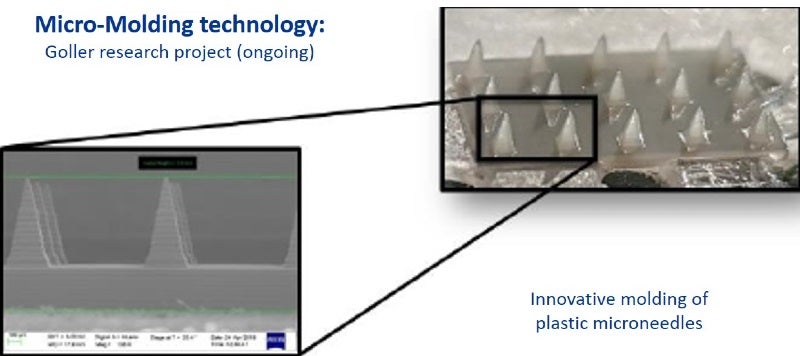
Micro-mould technology: we are equipped for moulding of high-precision micro-parts in a cleanroom environment, for challenging medical applications, like microfluidics, spray-tips, surgical and endoscopic devices.
The quality of our high-precision injection-moulded products for medical and pharmaceutical use is dependent on the tools we use. For this reason, GOLLER Systems highlights accurate service and maintenance of our injection moulds.
Cleanroom production
In our cleanrooms, following the International Organization of Standardization (ISO) class 7 and 6 and Good Manufacturing Practice (GMP) class C / B, we established the following portfolio of services:
- Printing, labelling including attachment of sterilisation point, assembling, US welding, gluing, filling and packaging
- Filling of liquid or solid media into smaller containers or syringes
- Packaging: soft and hard blister, special packaging for all types of sterilisation, in bulk, separate or as a complete system, storage and logistics, transfer to sterilisation
- Production of bags with one or more ports
We are also available as a reliable outsourcing partner and can seamlessly assume the production of existing products to absorb workload spikes.
Quality assurance
GOLLER’s utmost priority is the highest quality throughout. We reflect at 40 years of consistent top performance with a nearly 100% Supplier Performance Rating.
Our integrated quality system goes far beyond the fulfilment of official and legal requirements, certified according to international standards, such as ISO 9001, ISO 13485 (medical devices) and ISO 15378 (primary packaging for medicinal products).
Furthermore, our extensive risk management system and a reliably functioning change control process form the framework for successful and secure product development and production.
Extensive medical products and solutions
GOLLER’s portfolio ranges from single injection-moulded parts to complex tailor-made system solutions.
All projects are unique; our mission is the realisation of technical challenges for our customers, especially when there is no standard product available on the market.
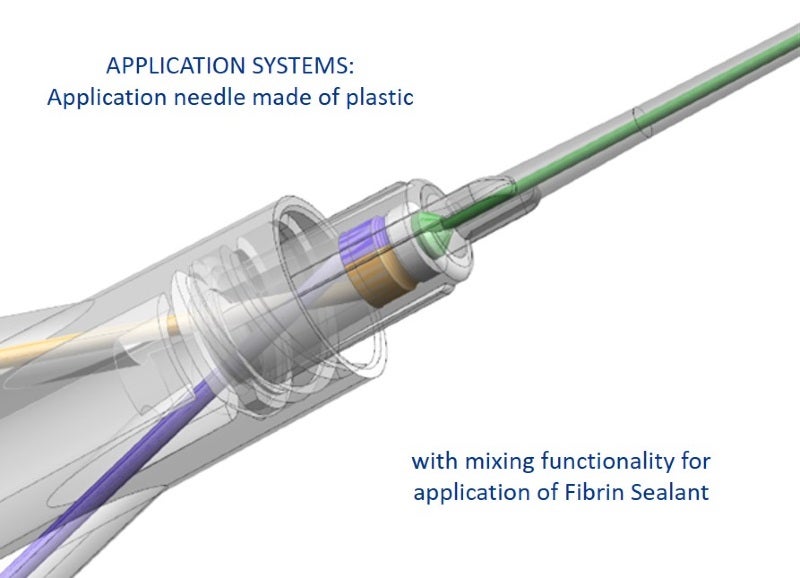
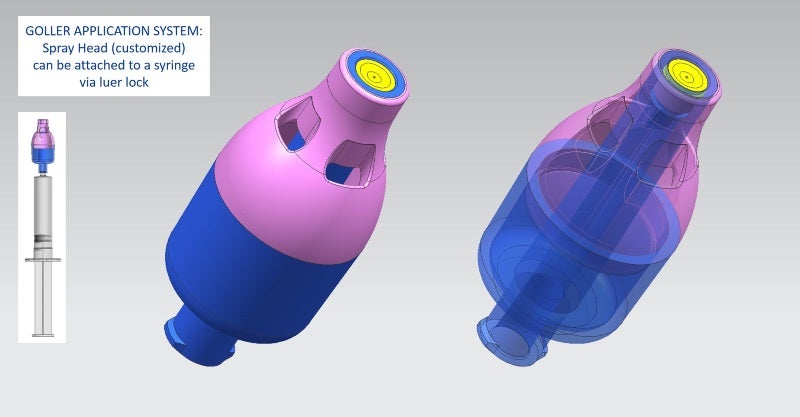
Examples for medical and pharmaceutical products include primary packaging, drug delivery systems, application systems (needles, spray head systems), bag systems with connectors, transfer and mixing systems (reconstitution, blood collection systems), microfluidic devices and nanostructures, and microneedle systems.
Examples for diagnostics include multi-allergy tests (test strip container) and coagulation disorder tests (housing for a test membrane).
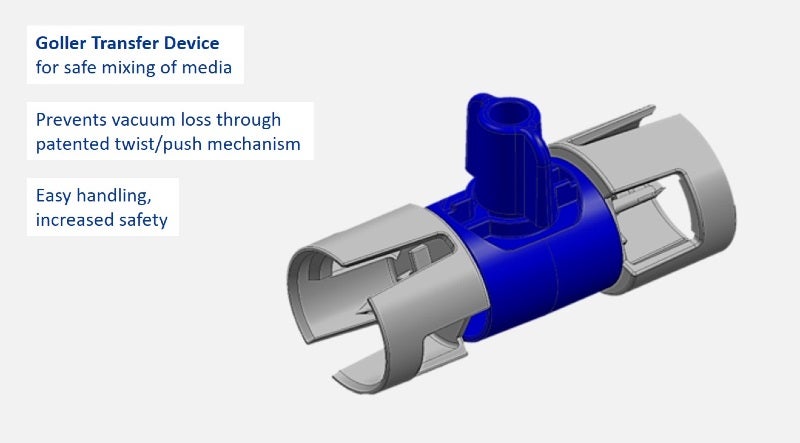
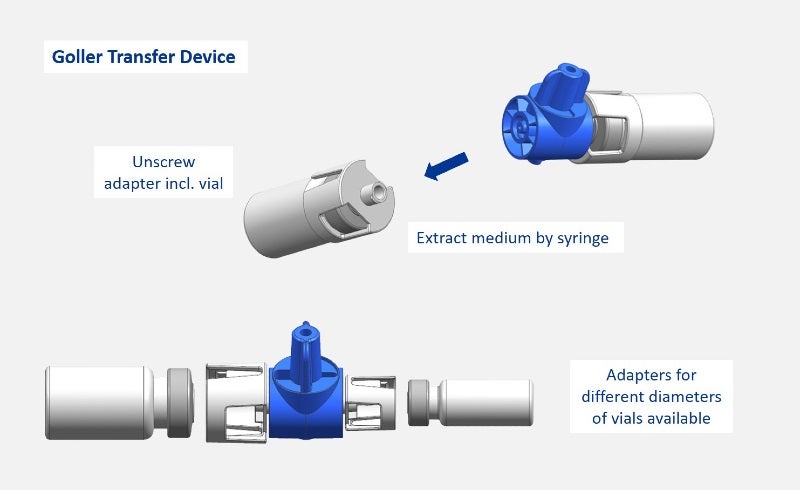
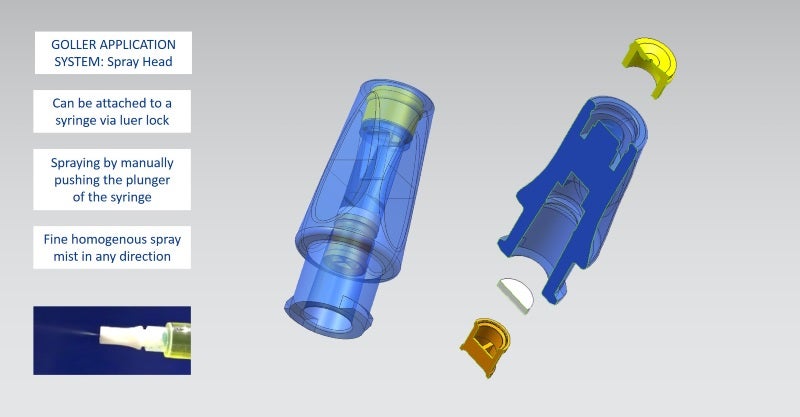
GOLLER has also developed an innovative transfer device for safe reconstitution of media, as well as a spray head system, which can be attached to syringes; both products are customisable.
Working with customers
GOLLER works with diverse types of customers such as start-ups, small and medium-sized companies, and global players in Europe and North America. We have successful partnerships with key customers for more than 40 years.
From the project idea through to serial production, you always have a personal contact partner from our committed development team at your disposal.
Due to our lean management structure, there is a significant competitive advantage for our customers: Quick reaction times, quick decisions and smart processes reduce the time in development and guarantee a fast time to market.
Our motto is ‘from vision to realisation’; we start supporting our customers from product conception to ensure we deliver the best solution available.