“Novel solutions for all senses”: Our passion drives us to develop and manufacture superior packaging solutions and applications for the pharma and medical industries.
With standard products, customised models tailored to customer requirements, and even new developments from scratch, we support our customers at every phase of the packaging development process using our intellectual property, which includes more than 100 patents and various cutting-edge polyolefin processing technologies.
Our more than 35 years of experience allow us to create innovative and safe products to meet the high demands of the pharma and medical markets. We focus on high quality, precision, and compliance in both development and production.
Primary packaging for the pharmaceutical industry – production capabilities and tooling shop expertise
As a trusted partner in the pharmaceutical industry, Gaplast understands the sector’s exacting standards and unique requirements. Our advanced production capabilities provide consistently high levels of quality for the following three distinct technologies, allowing us to respond flexibly to customer needs and deliver custom-developed solutions. Our coextrusion assets are specifically designed to produce multilayer AirlessMotion® bag-in-bottle systems, offering a broad range of sizes with custom options available upon request.
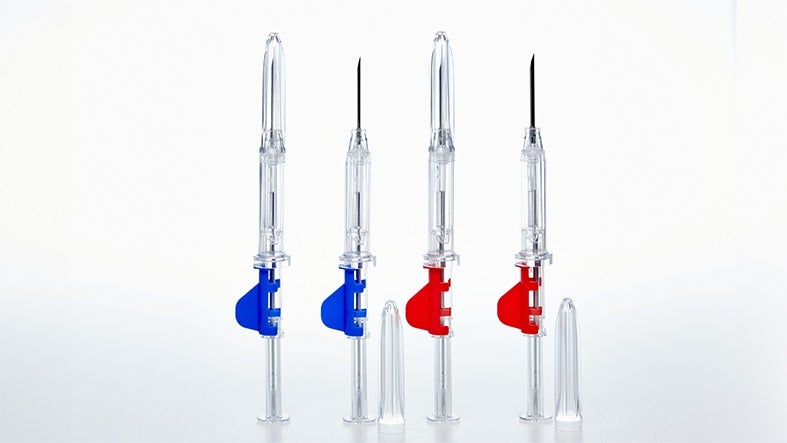
Our injection blow-molding machines can produce small-volume monolayer bottles with precise necks. Furthermore, our injection molding machines can produce a wide range of closure systems, from simple one-piece designs for effervescent tablets to complex, multi-component systems such as our implant injection system, known for its precision and repeatability.
Our tool shop and maintenance expertise allow us to produce small blow molds and small injection molds, prototype molds, and spare parts that meet high hygiene standards and industry requirements. Additionally, we adjust production tools as needed and provide on-site sampling. Our maintenance services ensure your equipment remains in excellent condition.
High quality standards for pharma packaging and medical devices
Quality is fundamental to all areas of our business, with clearly defined quality objectives and a commitment to constant improvement.
The system certifications of ISO 15378 and ISO 9001, as well as particle monitoring and microbiological control throughout production, ensure the consistency and reliability of our quality and production processes.
Qualification and validation are also crucial aspects of our quality assurance, with machines, tools, equipment, and production processes all thoroughly tested. We also offer guidance on sterilization processes and connect clients with appropriate partners.
Custom development as Gaplast’s extra plus
Gaplast specialises in creating innovative packaging solutions. Our diverse team of material researchers, developers, designers, project managers, and toolmakers ensures that we offer a comprehensive range of services, including idea generation, prototyping, serial production, and assembly, all tailored to meet our customers’ unique needs.
In addition, we have a network of partners and service providers who assist us in developing and manufacturing hygienic and sterile packaging and application systems.
Sustainability- a driver of innovation
Gaplast perceives sustainability as a driving force for innovation—an ongoing process. Consequently, we have addressed numerous initiatives and made significant progress in recent years. Prioritising sustainability has become integral to our business strategy. This includes developing practical packaging solutions, reducing emissions, and creating a positive work environment for our employees.
We are confident that our efforts will lead to an improved quality of life for all in the long run. While we are a globally oriented, family-owned business, we are also committed to making a substantial impact locally. Many of our ongoing initiatives directly align with specific goals outlined in the United Nations’ Sustainable Development Goals (SDGs), which we have incorporated into our internal action plans.