Digitalising Pharma Manufacturing
A fully digital and smart workplace offers many benefits, including lower costs, improved quality, and higher productivity. Nearly everyone agrees about that. Yet in pharmaceutical manufacturing, the pace of change has been slow.
Most companies still use at least some paper-based systems to guide and document processes. These entrenched and validated systems limit productivity and agility.
So, how can a life sciences company move from where it is today to a modern future? Companies envision future production where industrial internet of things (IIoT) enabled smart devices work with automation and data is always up-to-date, monitored and presented visually. This move to digitalisation was the major topic at this year’s Annual Pharma MESConference in Berlin.
Nymi was proud to showcase its technology there, along with major global pharmaceutical manufacturing execution system (MES) providers such as Werum, Rockwell, Siemens, and Emerson.
The Nymi Band was also on display at the Systec & Solutions booth showcasing a solution with its cleanroom IT equipment. Around 250 professionals assembled there for three days in late September.
So what can pharmaceutical manufacturers do to move forward? Clearly, implementing an MES solution is something that many companies are doing. Key drivers for using an MES are operational cost savings, productivity gains, quality improvements, and compliance through improved data integrity. There is immense value in producing more data with less data entry.
Yet because MES is a comprehensive set of software for managing plant operations, implementing and validating systems based on MES can take a long time. For companies with many products and plants, rolling out every MES function that they might need, to every line in a plant can take a while.
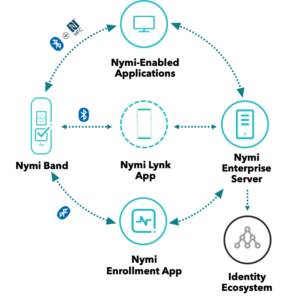
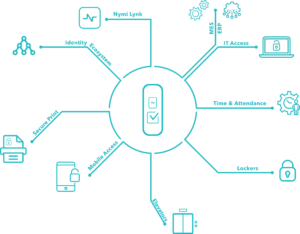
So how can you push for progress in your organisation? Including the Nymi Enterprise Edition in your MES rollout plan can offer very appealing productivity gains and streamlined processes, as well as improve compliance.
Let’s take an example of how process improvements that result from using Nymi Enterprise Edition can deliver significant productivity gains. Operators using an MES often must type their username and password 200 times a shift for logins and e-signatures. In this scenario, the Nymi Band could save each person two hours per shift.
Imagine replacing the frustrating experience of typing over and over again while wearing gloves and a gown with the seamless experience of tapping the Nymi Band against a reader to login or e-sign, without removing your gloves. Time and cost savings are substantial, while still complying with e-signature regulations such as the US Food and Drug Administration’s (FDA) 21 CFR Part 11.
The Nymi Band uses fingerprint biometrics to authenticate users, which supports cyber-security and reduces risk. For conference delegates, biometric authentication raised questions about how we handle the privacy of sensitive personal data. Nymi has designed its products with user privacy in mind. Biometric data is processed and stored securely on the Nymi Band. It is never uploaded to a server, and it is deleted when the user’s profile is wiped from the Nymi Band.
In addition to many intelligent questions about privacy, safety and compliance, Nymi received enthusiastic feedback about the productivity and process improvements that Nymi Enterprise Edition could achieve on a digitalised shop floor. Thank you to all of the MES vendors, IT specialists, MES project coordinators and operations managers that came to talk to Nymi at the show.
The company enjoyed exploring the digitalisation of pharmaceutical manufacturing and furthering its relationships with MES vendors.