FormPipe Takes SEK 2.5m Software Order from Recipharm
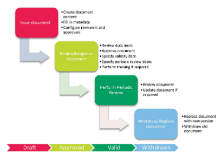
FormPipe Software has received a SEK 2.5m order from Recipharm for its electronic quality management system (EQMS), FormPipe Life Science. The software is designed to streamline quality and compliance processes.
Recipharm, which employs 1,700 people, is a renowned contract development and manufacturing organisation. The order will supply four out of the ten companies in the Recipharm group. The software will be implemented by FormPipe’s partner, Sigma.
Recipharm Strängnäs general manager Staffan Widengren said; "We believe that implementing FormPipe Life Science will drive efficiency in our operations by simplifying the processes for documentation and case management, which will add value to our customers."
Quality control is of the utmost importance in the pharmaceutical industry, as it involves every aspect of an organisation, from R&D through to manufacture and distribution. Companies must comply with strict industry regulation and Good Manufacturing Practice (GMP) quality standards, and will be inspected to ensure compliance. These requirements put a significant strain on IT infrastructure and support. FormPipe Life Science is designed to ensure regulatory compliance across your organisation without exhausting your IT resources.
FormPipe Software CEO Christian Sundin said; "This order from Recipharm shows that our investment in the life science sector now is beginning to transform into tangible orders. After being present on the life science market for some time, this order and the experiences we have gained during this time significantly strengthens our belief that we have a competitive and user-friendly offer to these companies."