Immune checkpoint inhibitor therapy has revolutionized cancer treatment in recent years and altered the prognosis for several cancers, most notably malignant melanoma and non-small-cell lung cancer.
Immune checkpoint inhibitors work by using the body’s own immune system to target tumours directly.
The immune system ‘turns on’ to attack potentially harmful agents; however, the immune system can also be ‘turned off’ to limit the immune response and prevent damage to healthy tissues.

Immune checkpoint inhibitors prevent cancer cells from turning off T cells
T lymphocytes (T cells) are a type of immune system cell that can kill cancer cells and have regions called receptors on their surface, to which other cells and molecules can attach and turn the T cell on or off.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSome cancer cells are able to bind to receptors on activated T cells and turn them off, but immune checkpoint inhibitors are highly effective drugs that are able to prevent cancer cells from turning off T cells, allowing T cells to infiltrate a tumour and prevent it from growing.
An influx of immune checkpoint inhibitors
The anti-CTLA-4 drug Yervoy was the first immune checkpoint inhibitor to reach the market, in 2011, and was followed by Keytruda, Opdivo and Tecentiq.
The combined revenue of immune checkpoint inhibitors is forecast to reach $38.6bn globally in 2023, accounting for 40% of all cancer immunotherapy revenue.
Despite commercial dominance, pipeline activity is low
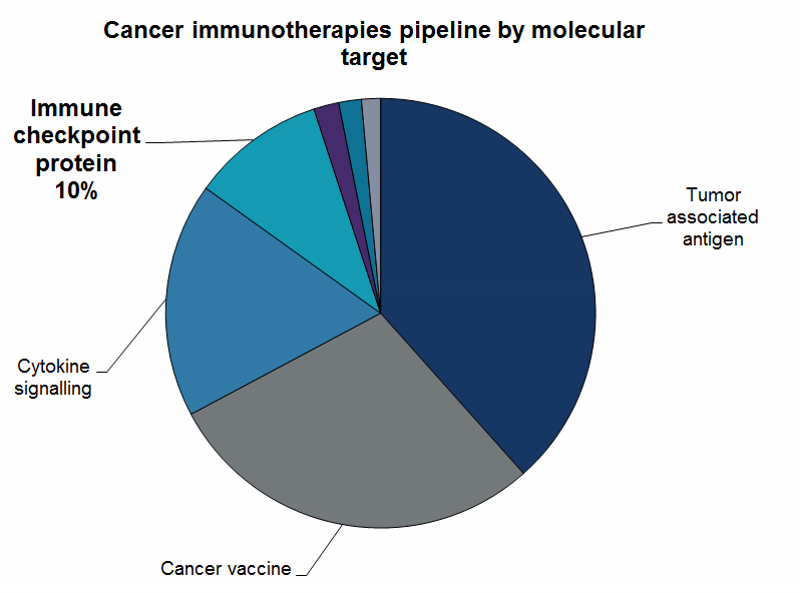
The number of immune checkpoint inhibitors in the pipeline increased from 157 in December 2016 to 219 in January 2018, which is just 10% of the whole cancer immunotherapy pipeline.
This can be expected from a class of therapy that is still emerging.
Despite the small pipeline size, the opportunities for growth within this group are encouraging, as treatments will inevitably cover more cancer indications, increasing patient numbers.