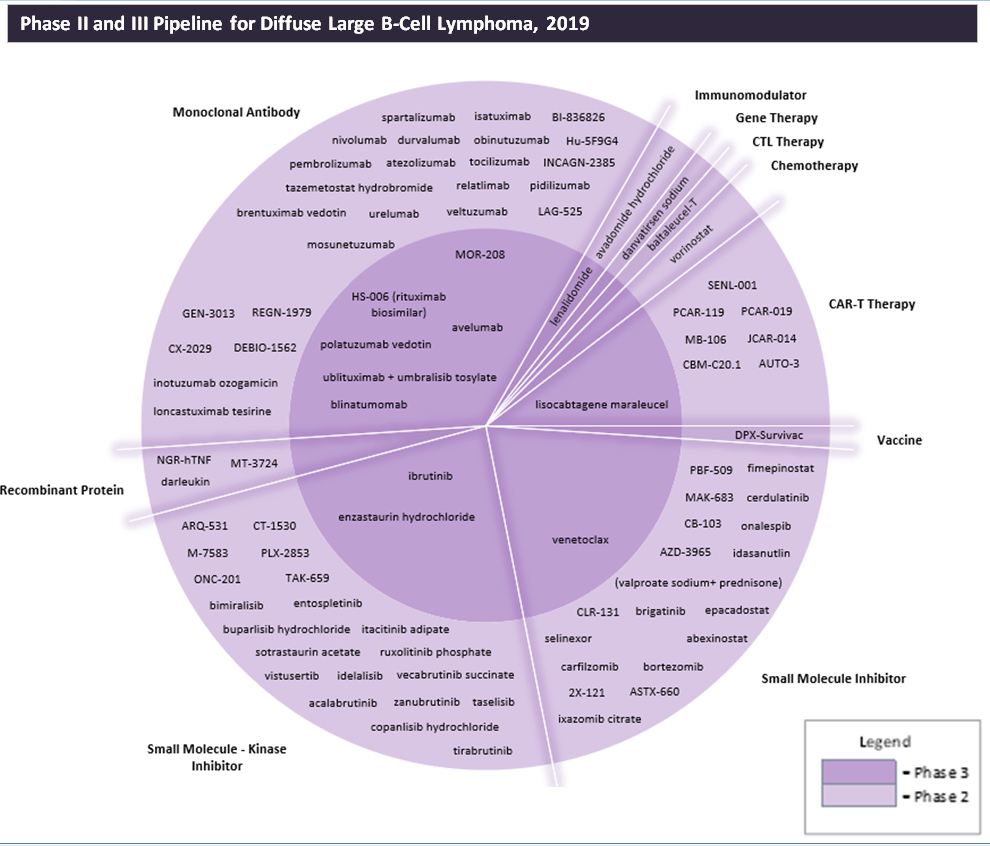
The extensive pipeline for diffuse large B-cell lymphoma (DLBCL), the most common subtype of non-Hodgkin’s lymphoma (NHL), is dominated by small molecule kinase inhibitors, which represent 47% of the therapies, followed by monoclonal antibodies (35%) and chimeric antigen receptor T-cell (CAR-T) therapies (8%). The prominence of CAR-T, a new and innovative therapy type, is due to the successful approvals that CAR-T therapies have already garnered in this indication. Kite’s Yescarta (axicabtagene ciloleucel) and Novartis’ Kymriah (tisagenlecleucel) received FDA approval in October 2017 and May 2018, respectively.
Because these CAR-T approvals were based on Phase II trials, the current Phase II CAR-T therapies may also be very close to approval. The current front-runner to achieve the next CAR-T approval is Celgene’s lisocabtagene maraleucel (JCAR017), a therapy that the company is positioning as potentially being best in class. Celgene claims that lisocabtagene has a better safety profile than other CAR-T therapies, although key opinion leaders (KOLs) believe that it is too early to confirm these differences. Another very promising Phase III agent that may be close to approval is Roche’s polatuzumab vedotin, which was granted priority review by the FDA earlier this month.
Source: GlobalData

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData