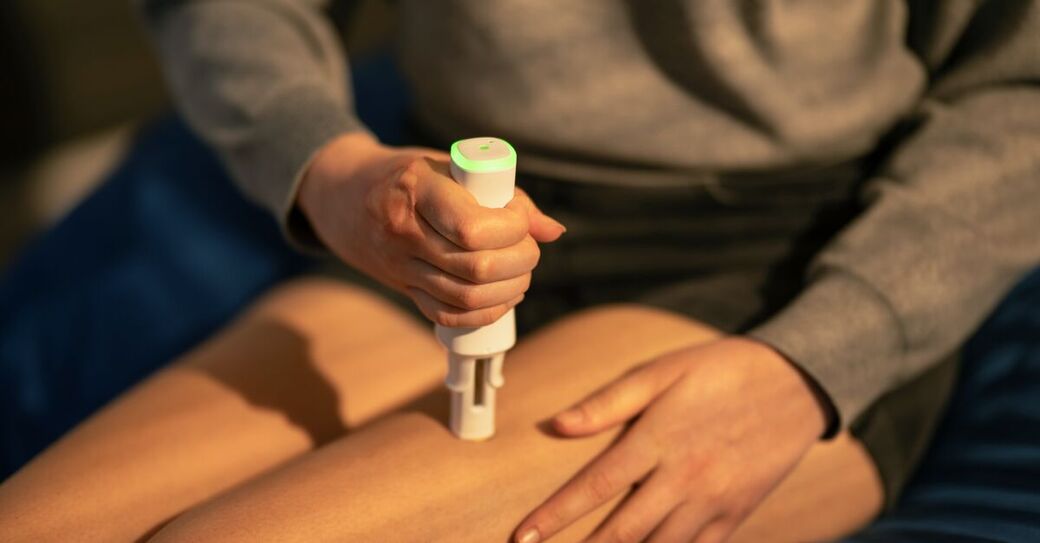
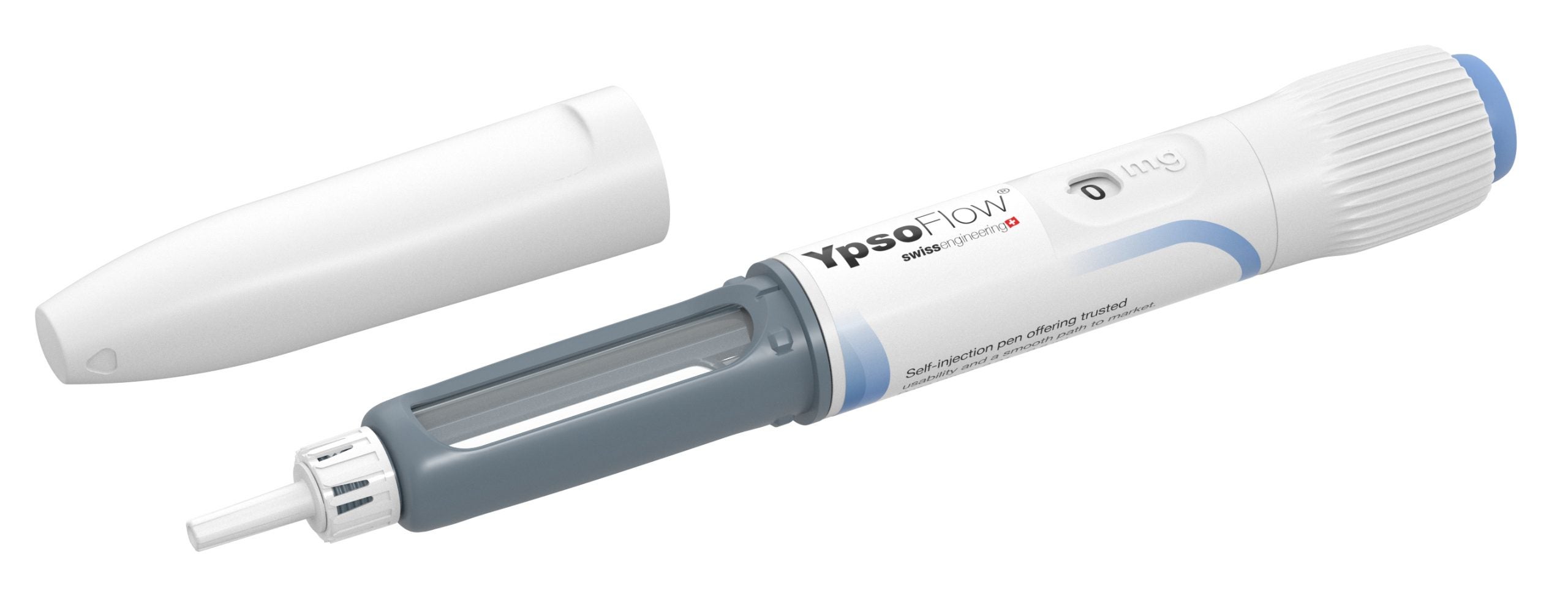
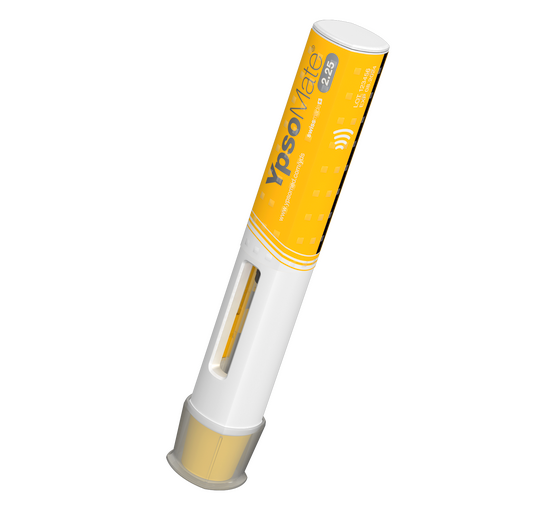
Ypsomed is the leading developer and manufacturer of injection systems for the self-administration of liquid medication. As the reliable partner of pharmaceutical and biotech companies for more than 40 years, Ypsomed provides tailored self-care solutions to meet specific needs.
The company’s proven track record attests to its pioneering portfolio of pens, autoinjectors, and wearable devices for pharmaceutical applications.
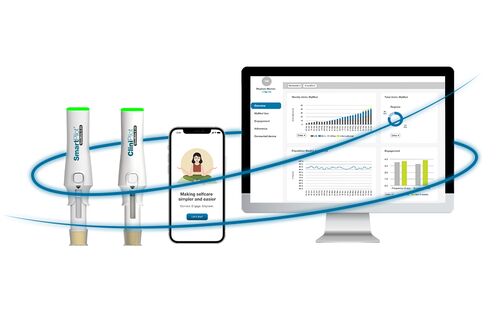
Complete with best-in-class features and fully integrated with digital health services, Ypsomed’s solutions make therapy easier and contribute towards improving the quality of life for millions of people with chronic conditions.
Modular self-injection platforms for pharmaceuticals
Ypsomed’s self-injection products offer speed, reliability and versatility. Each product helps support development and customer project risk mitigation, while being completely scalable across the whole clinical chain, from early trial stages all the way through to commercial launch.
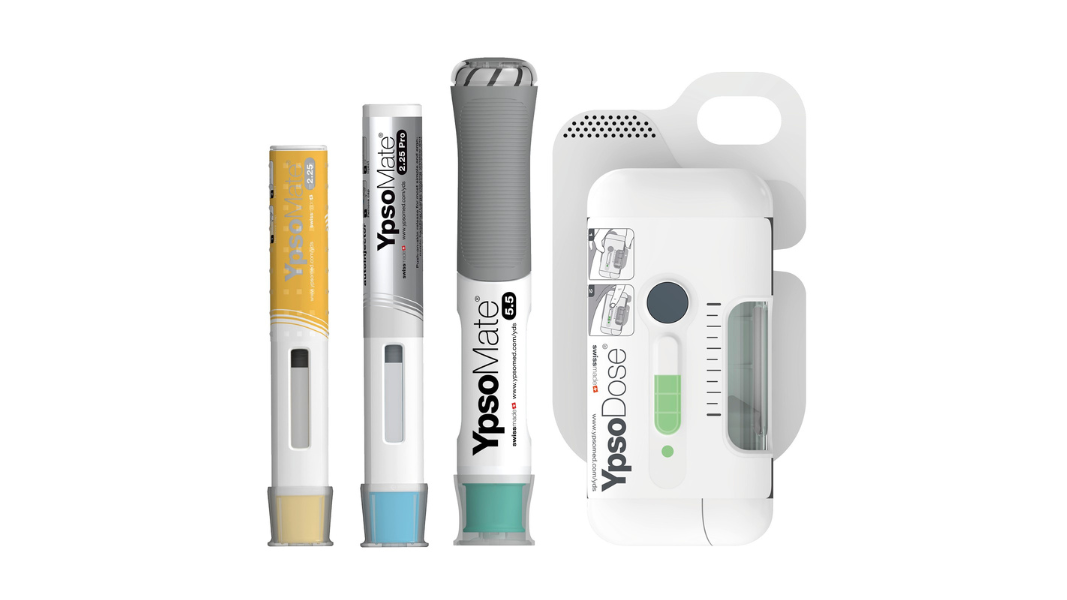
With a fully comprehensive product portfolio that is leading the market, Ypsomed’s offerings include YpsoMate and UnoPen.
Our modular platforms are thoroughly developed and tested, as well as validated through designs, pre-approved parameters, and a proven industrial manufacturing infrastructure. This promotes trust for our customers and a tried and tested foundation that saves time and leads products to market faster and more effectively.
Ypsomed platforms are designed for a wide variety of drug formulations for different viscosities and fill volumes. This flexibility is inherently built and allows for customisation without changing the main device.
Building strategic partnerships
Ypsomed has been a trusted self-injection device supplying partner to our pharmaceutical customers for more than 40 years. Over the last two decades, we have seen our product offerings evolve from cartridge-based peptide therapies for pens to also include syringe-based autoinjector therapies that answer the special needs of pharmaceutical companies and patients for antibody-based treatments.
While Ypsomed strives to be a leader in self-injection devices, we partner with these key suppliers to ensure that the delivery of the drug–device combination product is prompt, on time and meets the required needs of companies and patients.
Through strategic partnerships, our customers in the pharmaceutical industry benefit from top-quality solutions that reduce costs and reduce timelines for each drug product phase.
Drug delivery manufacturing processes
Ypsomed offers more than manufactured drug delivery products; we infuse our expertise into our solutions, optimise device platforms for patients and drugs, and provide global scalability with a goal towards ongoing and sustainable manufacturing excellence.
From clinical trial stages all through to full-scale commercial production, we instil confidence in our customers and offer unmatched quality that helps us build trusted partnerships.
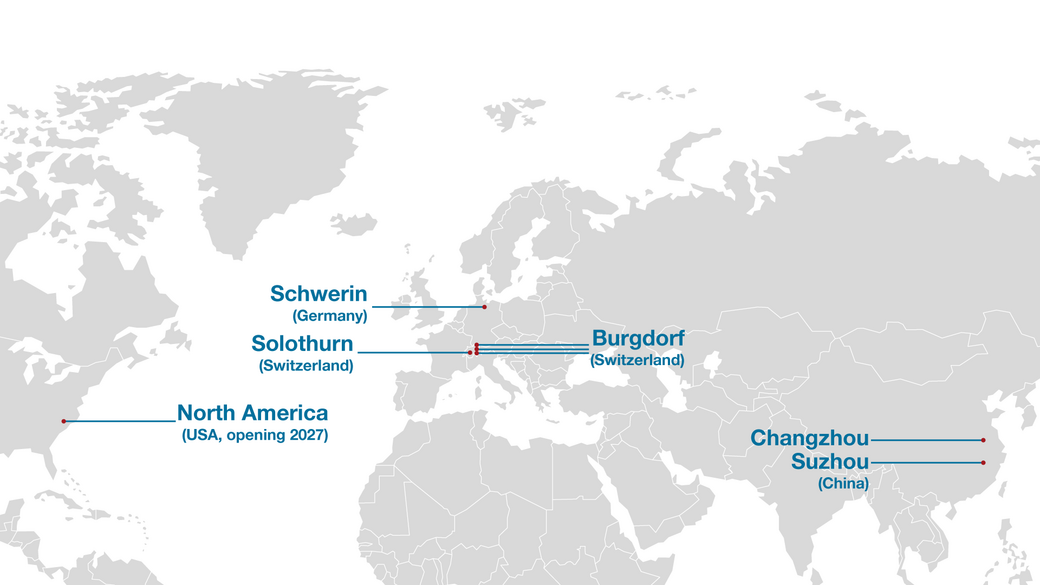
All key manufacturing processes are performed in-house, and these range from tool design and manufacturing to injection moulding and assembly. Our internal manufacturing operations are located at our head offices in Burgdorf, Switzerland, and facilitate high efficiency and seamless communication among teams for the best possible results. Fast communication, prompt feedback and full control help ensure products are delivered on time and to the highest standards.
Additional services for every stage of development
Our services expand beyond drug delivery devices to also include built-in and optional services for every developmental stage. From human factors and regulatory expertise to sustainability and testing, we help minimise risk and support long-term market success through reducing challenges and driving forward customer launch.
Our services help accelerate the full lifecycle of combination product development, which includes early planning, customisation, industrialisation, launch and post-market assistance.
There is seamless collaboration among professionals with expertise in design, usability, quality, regulatory, and testing, which helps advance decision-making, better communication, and harmonious alignment from design to delivery.
Ypsomed sustainability values
Ypsomed is committed to a long-term corporate sustainability strategy, and its ethics revolve around responsibility towards our employees, our partners, society and the environment.
The increased use of plastics associated with the growing adoption of pre-filled self-injection systems has an environmental impact. In response, Ypsomed has established responsibility & engagement as a fully integrated pillar of its corporate strategy.
Our commitment to sustainability is multifaceted and encompasses the company’s NetZero Program, our roadmap to net zero emissions by 2040, as well as our ecodesign ethics that see eco-friendly practices for our product design cycles.
Looking ahead, our next generation of platform products will mark a major shift towards sustainable self-injection device manufacturing that is responsible and sensitive to the environment. Our award-winning autoinjector, YpsoLoop, which was launched in October 2025, allows for material recovery through automated disassembly and recycling.
About Ypsomed
In 1984, brothers Willy and Peter Michel founded Disetronic in Burgdorf, Switzerland. The company was the first to introduce a micro insulin pump to the market and, along with its infusion systems, also specialised in injection systems.
In 2003, co-founder and majority shareholder Willy Michel sold Disetronic’s infusion part of the business to Roche, but kept the injection business, which was how Ypsomed was born. Since then, Ypsomed has grown to be successful in the pharmaceutical market.
Ypsomed is headquartered in Burgdorf, Switzerland, with various manufacturing facilities around the world. The company employs more than 2,000 people who are committed to making self-care easier and more sustainable.