Biopharma Group
CDMO Partner for Pharmaceutical and Life Science Projects
Biopharma Group partners with pharmaceutical companies to help bring their projects to market quickly and cost-efficiently.
Subscribed
You have successfully submitted your enquiry. Someone from our company will respond ASAP
About Us
At Biopharma Group, our primary focus is to deliver the highest standard of service, support, and products to our clients. With a customer-centric ethos and culture, we strive to meet the precise needs of your projects regardless of their size or stage. Our mission is to augment your in-house expertise to ensure successful outcomes every time.
By working closely with our clients, we gain a deeper understanding of their unique requirements, enabling us to provide tailored solutions to meet specific needs. We believe that by augmenting your team’s capabilities, we can make each product project a resounding success.
Contract development and marketing organisation services
Biopharma Group’s research and development (R&D) consultancy and lab analysis division was established to provide unbiased contract research, analysis and development services, as well as training and analytical instrumentation on an international scale.
Our services have expanded to include contract manufacturing for lyophilisation (lyo) and liquid formulations, which have proven particularly beneficial for the diagnostic and vaccine sectors. We also offer spray drying for more robust formulations to suit clients’ needs.
We are due to open a dedicated good manufacturing practice (GMP) facility in 2023 and will be expanding our existing cyto and highly potent active pharmaceutical ingredient (HPAPI) suite between 2023 and 2024.
Analysis of dried products and liquid formulations
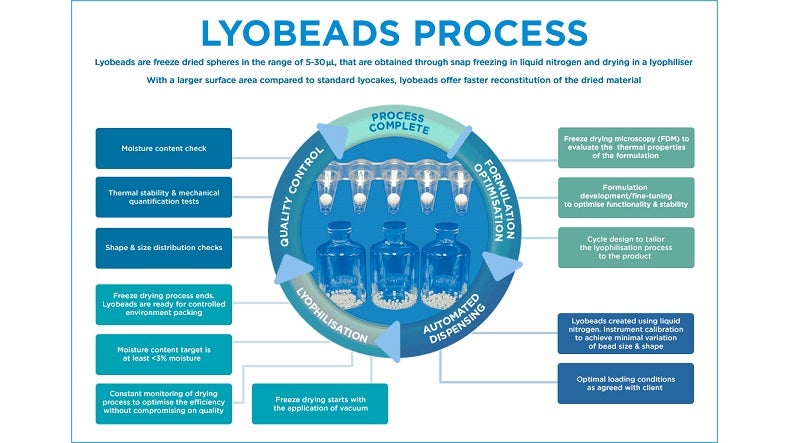
Biopharma Group’s in-house lab and scientists in Winchester, UK, are dedicated to dried product and liquid formulation analysis. We specialise in the freeze-drying stages of product development, using either traditional lyocakes in vials or lyobead processing for suitable products. Our contract R&D services cover pre– and post-lyophilisation or spray drying testing, including pre-process comparable trial cycles to establish the best processing method for your product.
To facilitate real-time data generation, we have incorporated process analytical technology (PAT) into our freeze dryers. This technology is applicable across various industries, including pharmaceuticals, nutraceuticals, in-vitro diagnostics (IVDs), biotechnology, and organic materials.
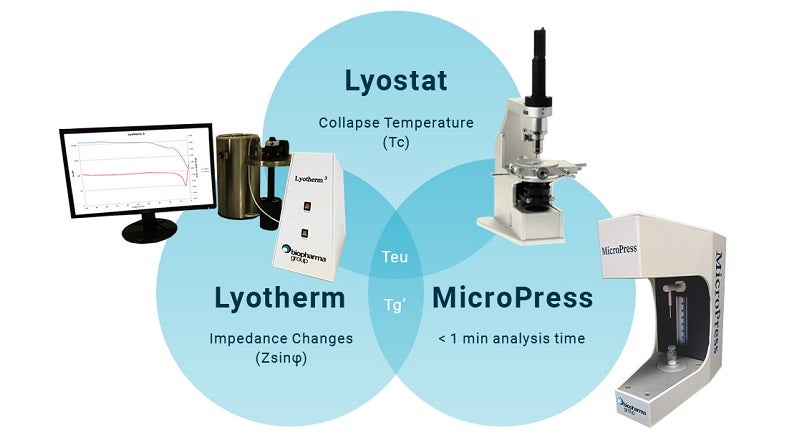
Our range of services includes pre-formulation studies, formulation development, cycle development, process reviews, product characterisation, sample production (including IVDs and vaccines), scale-up/optimisation, and specialist post-process analysis using advanced instruments like the MicroPress.
Saving time and money by improving process efficiency
By choosing Biopharma Group as your CDMO partner, you can benefit in numerous ways, including:
- Cost reductions: Proof of concept studies ensure that the best processing method is used for your desired results, increasing efficiency while reducing costs and batch rejections.
- Optimised cycles: Our expertise can significantly reduce cycle time, saving you valuable resources. Based on case studies, we have achieved time savings ranging from two to five days.
- Saving time: We can help optimise your formulation, reducing production time by 60% to 80% through improved collapse temperature and reduced primary drying.
- Reducing the percentage of rejections per cycle: Our services have led to significant reductions in rejection rates for customers, minimising wastage and achieving rejection extents of less than 2% per batch.
- Recovered efficiency in terms of cost and time: By optimising processes, we have helped clients recover significant amounts, ranging from $30,000 to $50,000 for each day saved.
- Extended shelf life: Products that have undergone long-term stability studies and have proved their stability for more than five years, with shelf lives exceeding 24 months.
Customisable research and development consultancy services
In addition to our core offerings, Biopharma Group provides customisable R&D consultancy services. Our collaborative development work allows us to evaluate new advances and co-develop new products in various fields, such as wound management and regenerative medicine.
By leveraging our freeze-drying expertise, we can offer valuable insights for all stages of pharmaceutical development. We also have a Class 7 cytotoxic handling suite available for assisting in cytotoxic developments, with a dedicated GMP unit currently in development.
Lyophilisation training courses for freeze-drying
To ensure ongoing success for clients with their own freeze-drying facilities, Biopharma Group offers expert-led freeze-drying training courses. Led by our specialist tutors with extensive practical lyophilization experience, these courses cover the challenges of operating an efficient and robust freeze-drying process.
We offer regularly scheduled lyo training courses in the UK, Europe and the US, as well as customised courses (tailored to your requirements) that can be conducted on-site at our facility in Winchester, UK, or by live webinars. We also do introductory webinars, accredited micro-courses and pre-recorded video modules that can be bought in bundles or individually.
International divisions to serve overseas clients
Biopharma Group has several divisions that cater to specific territories and needs. Our Equipment Sales & Technical Service unit is a leading supplier of equipment for the pharmaceutical, biotechnology, and process industries in the UK, Ireland and France, bringing years of expertise in the processing sector.
Biopharma Technologies France (BTF) offers a combined proposition of equipment sales and expertise from our CDMO division, which is ideal for our French-speaking clients. For customers in the US, Biopharma Technology LLC focuses on providing analytical instruments, CDMO services, and training courses.
Contact Details
Website
Email Address
Address
Winnall Valley Road,
Winchester,
SO23 0LD,
United Kingdom