US-based nuclear technology company SHINE Medical Technologies is building a medical isotope production facility in Janesville, Wisconsin.
In 2013, the company submitted a construction permit application for the plant, including environmental and preliminary safety reports, to the US Nuclear Regulatory Commission (NRC). In February 2016, the NRC approved the plant’s construction.
SHINE will use the facility to produce molybdenum-99 and other medical isotopes used in medical imaging procedures. A ground-breaking ceremony for the facility’s construction was held in May 2019.
SHINE medical isotope production facility background
Molybdenum-99 (moly-99) is a radioisotope used in medical diagnostic procedures. Over the coming years, most of the nuclear reactors that produce moly-99 will be closed. These ageing reactors have led to isotope shortages, impacting patient care.
Though the US accounts for around 50% of the demand for moly-99, it depends on imports from foreign nuclear reactors. In order to reduce the country’s reliance on trade, SHINE aims to produce cost-effective and environment-friendly medical isotopes domestically in the US without using highly-enriched uranium (HEU).
Medical isotope production facility design details
The facility will consist of five buildings covering a 91,000ft² area in total. This will include the main production facility, equipped with eight sub-critical irradiation units, and a radioisotope production facility with three hot cells for moly-99 separation and purification.
The facility will also include waste staging and shipping capabilities, an administrative building, a diesel generator, and other ancillary infrastructures such as storage sheds, storage tanks, parking lots, and water tanks.
Construction of the SHINE medical isotope production facility
The construction of Building One of the medical isotope production facility began in August 2017 and was completed in February 2018.
The 11,400ft² building accommodates an integrated, full-size SHINE production system that will be used to train employees while the main production facility is being constructed.
Building One will also be used as a technology development centre once the main building is completed.
Production technology at the SHINE medical isotope production facility
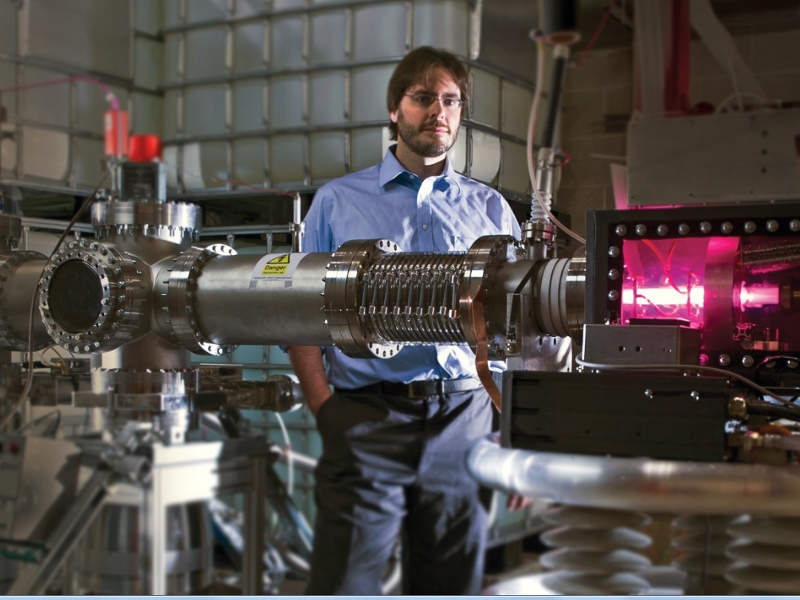
SHINE will deploy a patented non-reactor-based technology, developed in partnership with Argonne National Laboratory and GE Healthcare in November 2015, at the facility.
The technology uses a sub-critical fission process in irradiation units to manufacture medical radioisotopes. The main components of the irradiation units include a neutron driver, a sub-critical operating assembly, a light-water pool and surrounding biological shielding.
Deuterium ion source contained in the neutron driver is accelerated into a tritium gas target chamber to produce neutrons, which fire upon a target solution contained in the sub-critical operating assembly to produce radioisotopes.
The light-water pool provides shielding for the target solution and acts as a neutron reflector. The process is cost-effective, uses less electricity and produces less waste.
Products details
The Janesville facility will primarily produce moly-99 radioisotope to generate technetium-99m (tech-99m), which is used in more than 40 million medical imaging procedures each year. Tech-99m is used to conduct stress tests to diagnose heart disease and bone scans to determine the stage of cancer progression.
The facility will also produce cancer therapy iodine-131 and xenon-133, used to diagnose lung disease.
Contractors involved
In September 2017, US-based construction company Baker Concrete Construction was contracted for the construction of the medical isotope production facility. The construction of Building One was carried out by construction firm JP Cullen.
Marketing commentary on SHINE Medical Technologies
SHINE Medical Technologies was established in 2010 with a focus on producing radioisotopes for medicinal purposes. The company is headquartered in Janesville, Wisconsin, US.
In 2014, SHINE entered moly-99 supply agreements with GE Healthcare and Lantheus Medical Imaging. The company is also evaluating potential sites for developing a second facility to serve the Asia and Middle Eastern markets.