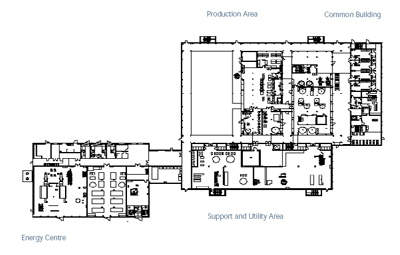
The NovoSeven pharmaceutical facility in Hillerød, Denmark was constructed by NNE (Novo Nordisk Engineering), the engineering subsidiary of Novo Nordisk, well-known for its fast track life science construction projects using modular engineering. The facility was named the best in the world in the 2005 Facility of the Year Awards sponsored by the International Society for Pharmaceutical Engineering (ISPE). This was the first time the award was given.
The 14,000m² (150,695ft²) facility on the 163,000m² site, which is believed to have cost an estimated DK800m (US$143m), was constructed for the production of the NovoSeven clotting factor for the treatment of patients with Haemophilia A or B. The facility was created as an integrated production plant for mammalian cell fermentation, recovery and purification.
The facility was handed over to Novo Nordisk in the fourth quarter of 2002 for technical inspection and the start of validation; the plant came into full production by the end of the second quarter of 2003.
The facility, which employs over 1,500 personnel, doubled the production capacity of NovoSeven for Novo Nordisk.
WHAT DOES THE PLANT PRODUCE?
The Hillerød facility works on researching, developing and producing medical devices for the treatment of diabetes and haemophilia. The site also has warehouses for raw materials and products, an energy plant and administration. Activity includes:
- Research and development in protein delivery systems. Protein delivery systems include insulin pens, dosers and other systems for delivering pharmaceutical products. Novo Nordisk’s pens and dosers for administration of insulin are developed in Hillerød.
- Manufacture of pre-filled insulin devices. NovoLet and FlexPen are used for administration of insulin. These products are manufactured using injection moulding for the plastic components. The insulin is then filled into cartridges, which are assembled and packed.
- Manufacture of durable pens. NovoPen is a system used for administration of insulin. The components are manufactured externally and then assembled using automatic and manual processes.
- Production of rFVIIa, the active ingredient in NovoSeven, a preparation for treatment of haemophiliacs with inhibitors against conventional haemophilia medicine.
CONSTRUCTION
NNE was able to complete the state-of-the-art glass and steel NovoSeven production facility in only 18 months by breaking down the construction process into modules. Fast-track implementation of this kind is critical to the pharmaceutical industry where it is essential to establish manufacturing capacity as soon as a product has been approved by the authorities.
The construction process included comprehensive parallel construction of independent process modules and off-site testing of each process module. The architect for the facility was NNE (the NovoSeven facility was designed as a compact facility consisting of one big hall). The process design and engineering was carried out by NNE.
Construction was carried out by MONNET, a joint venture between NNE and MT Højgoerd. Equipment suppliers for the facility included BBI, Millipore, Christ, Semcon, Bravida and Emerson Process Management. The commissioning and validation consultant for the facility was NNE. The number of production staff employed at the facility is 100 and the number of production vessels is 81. The automation system is in full compliance with CFR 21, part 11.
MODULES
The modules were divided into five subgroups: cell fermentation, chromatographic separation, raw materials and buffers, clean utilities and facility utilities. Each group of modules was ordered from a supplier with expertise in the specific type of equipment.
The modules were ordered as complete functional, operational units, including instrumentation, power panel for motor starters, automation panel with DCS controllers and I/Os. The modules only needed to be connected to the piping, to power and to a fibre optics communication cable in order to be in operation.
To obtain a degree of standardisation throughout the facility, the process control system and critical component manufacturers were fixed and there were a number of mandatory design and construction standards. Apart from that, the suppliers were free to use their preferred components and methods.
Using this project plan NNE got what was needed, with a good balance between easy maintenance and the lowest possible investment cost. The module suppliers were obligated to order all process control software from a predefined supplier, based upon the supplier’s own functional specifications. A strict S-88 compliance was enforced, in order to make the concept possible.
CONSTRUCTION OF MODULES
Each module was independent and self supporting from a constructional point of view, which meant that supports for piping, cabling, power panel, automation panel and instrumentation were an integrated part of each module. All module suppliers chose to use RHS profiles for the construction of the module frames, which ended up giving a ‘clean lines’ impression in the finished facility. A bonus from the use of this method was that all cables on the modules could be run inside the RHS profiles and thus
hidden.
The modules including very large vessels reaching through the deck were constructed with the tanks separately and a skid at the top and at the bottom to support the piping and the components. Those modules were fully assembled in each supplier’s workshop and then disassembled for transportation.
The medium sized modules were constructed on a bottom frame, closed with stainless steel plates with RHS frames and transported in one piece. The longest of these was 12m with seven pressure vessels. After installation, the gap between the floor and the bottom frame was sealed using epoxy resin. The smallest modules such as seed fermenters and the final purification steps were constructed on RHS structures on adjustable legs.
NOVOSEVEN
NovoSeven (FVIIa) is an essential life-saving clotting factor that enables blood to coagulate. The drug was developed by Novo Nordisk and approved for treatment of bleeding of inhibitor patients – patients with haemophilia A or B who have developed antibodies against coagulation factors VIII or IX that are normally used for treatment of haemophilia patients. For these patients there are no alternative treatment options.
Novo Nordisk completed the compilation of the regulatory dossier related to the use of NovoSeven in blunt trauma in 2001. Novo Nordisk has manufactured NovoSeven since 1990.
NEW PILOT PLANT FOR BIOLOGICS
In June 2007 Novo Nordisk inaugurated a new pilot plant for biopharmaceutical products in Hillerød. The new plant will be used to develop and manufacture new biopharmaceutical products based on proteins cultured in mammalian cells. This is an area that Novo Nordisk has been trying to expand into.
The pilot plant required an investment of DKK350m (€47m) investment and consists of a 3,500m² area with full development and production facilities. The pilot plant has been used for the development of investigational compounds including antibodies for cancer therapies.
Novo Nordisk would like to establish a foothold in the areas of oncology and inflammation. The company already has two cancer therapy compounds in clinical trials. these include interleukin 21 and interleukin 31 (still in preclinical). Novo Nordisk has been developing interleukin 21 in conjunction with ZymoGenetics of Seattle.
ZymoGenetics was spun off from Novo Nordisk in 2000 and seven ZymoGenetics compounds were licensed to the parent company including interleukin 21 and interleukin 31. Novo Nordisk has now returned four of the seven compounds to ZymoGenetics, including interleukin 31.
As of March 2008 Novo Nordisk announced it was pulling out of further development work on interleukin 21 and interleukin 31 and will out-license interleukin 21 to Merck Serono (Novo Nordisk holds rights to the drug outside North America). Novo Nordisk’s oncology programme includes five clinical trials, four of which involve treating various types of cancer with interleukin 21.
Novo Nordisk now intends to stop any further research into oncology and instead concentrate future efforts into inflammatory disease. Interleukin 31 was being developed as a treatment for inflammatory disease but the collaboration in its development is still being ended with no reason given for the decision. Economic reasons are the most likely. The fate of the relatively new pilot plant at Hillerød is unknown.
BIOTECHNOLOGY DEVELOPMENT
Dutch biotech Crucell licensed its STAR technology to Novo Nordisk in January 2007. Novo will evaluate the technology for the production of monoclonal antibodies (MAbs) using proprietary mammalian Chinese hamster ovary (CHO) cell lines. STAR technology increases production of recombinant antibodies and therapeutic proteins on mammalian cell lines. The cell clones created by the STAR process produce five to ten times more antibody or protein than clones produced by other processes.