OncoMed Pharmaceuticals, a clinical stage company developing medications that target cancer stem cells (CSCs), has confirmed that the United States Patent and Trademark Office has issued a patent (Patent No. 8,507,442) for its methods of treating cancer with its antibody vantictumab (OMP-18R5).
Vantictumab specifically targets and prevents the Wnt pathway, which is a protein-based signalling pathway in cancer that passes information from outside of a cell to its interior.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It was originally identified at OncoMed from the HuCAL GOLD® antibody library (MorphoSys AG) by attaching itself to the Frizzled-7 receptor.
The patent is set to expire in 2029.
Vantictumab has successfully shown anti-CSC and anti-tumour activity in tissue taken from patients and it is currently being evaluated.
The test has provided preliminary signs of single-agent activity in patients with neuroendocrine tumours.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to Paul Hastings, chief executive officer of OncoMed, “OncoMed’s new patent, together with its composition-of-matter patent, provides key patent coverage for vantictumab.”
He added: “The issuance of this patent also further validates the novelty of this promising antibody and the innovative nature of OncoMed’s ongoing discovery programmes, which have not only produced vantictumab, but also four other anti-CSC agents currently in clinical trials.”
The Vantictumab vaccine is part of OncoMed’s collaboration with Bayer Pharma.
The company receives funding from Bayer for its partnered programmes. It also depends on the development and marketing efforts of its partners in order to ensure the commercial success of its partnered product candidates.
OncoMed has subsequent patent applications for vantictumab in 14 other countries in addition to Europe.
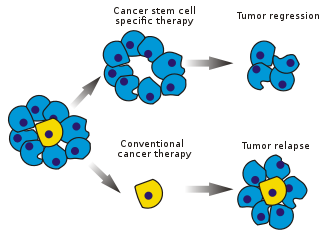
Image: Stem cell specific and conventional cancer therapies. Photo courtesy of Peter Znamenskiy.




