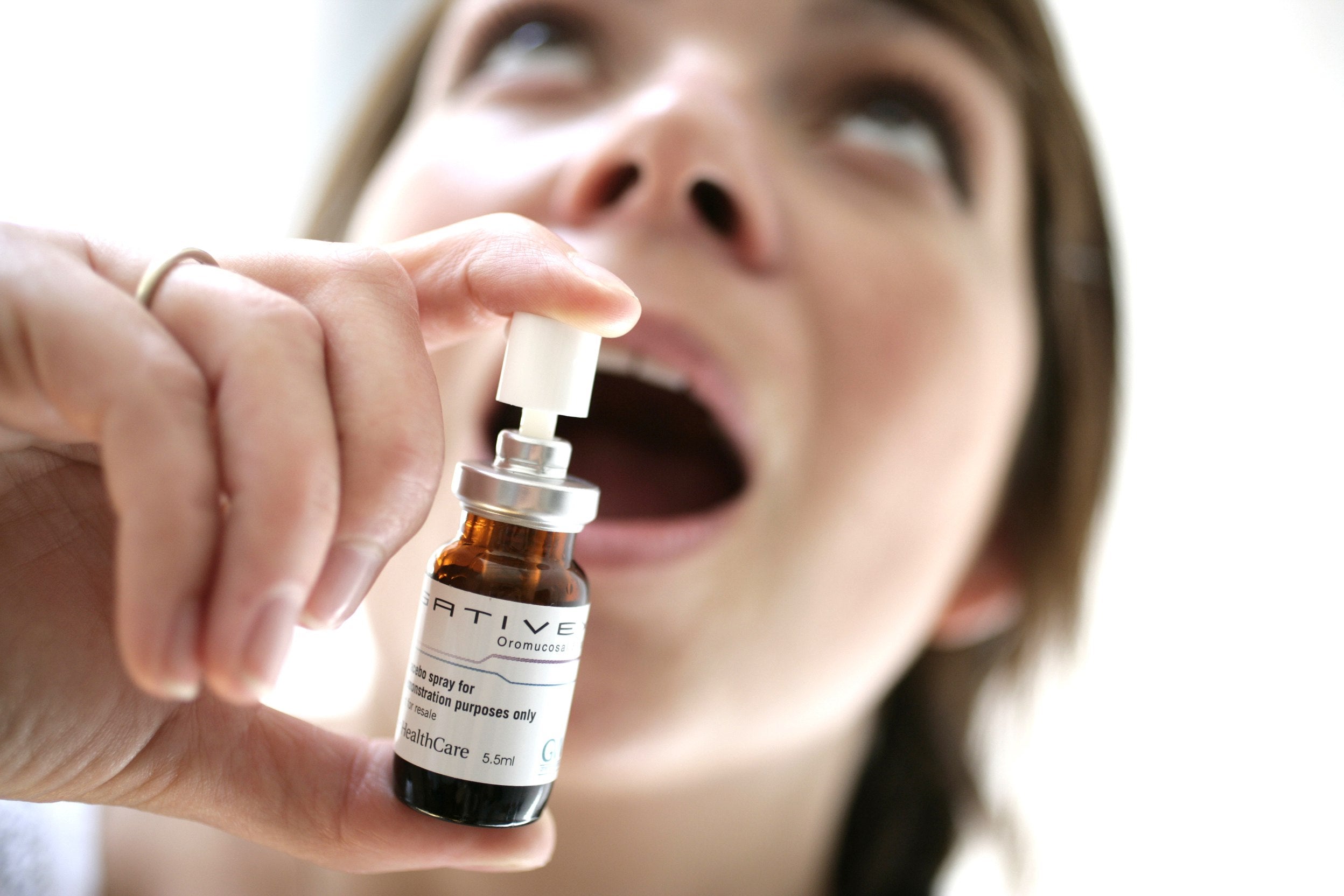
GW Pharmaceuticals has received full marketing authorisation from the Swiss authorities for its prescription medicine Sativex to treat moderate to severe spasticity in multiple sclerosis (MS) patients who have not responded to other medications.
According to the GW, the launch timing of the medicine is dependent on completion of pricing and reimbursement procedures.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company’s European partner, Almirall, will be responsible for the commercialisation of Sativex in Switzerland.
Spanish pharmaceutical company Almirall is the exclusive distribution partner for Sativex in the European Union (excluding the UK) and the EU accession countries, as well as Switzerland, Norway, Turkey and Mexico.
GW chief executive officer Justin Gover said: “We now look forward to working with our partners, Almirall, towards this launch to enable MS patients in Switzerland to benefit from this important new treatment.”
The medicine is currently approved for use in the treatment of MS spasticity in 23 countries, including 17 countries in Europe.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPreviously, regulatory authorisation has been received in Belgium, the Netherlands, Portugal, Czech Republic and Slovakia, with launches expected to start from the end of this year.
Sativex is available on prescription in the UK, Spain, Germany, Canada, Denmark, Norway, Israel, Austria, Poland, Sweden, Italy and Finland with launches currently in preparation for a further eight European countries, as well as Australia, New Zealand and Kuwait.
The medicine is also in Phase III clinical development as a potential treatment of pain in people with advanced cancer.
The trial is intended to support the submission of a new drug application (NDA) for Sativex in cancer pain with the US Food and Drug Administration (FDA) and in other markets worldwide.
Sativex is administered orally and is composed of two principal cannabinoid components, cannabidiol and delta-9 tetrahydrocannabinol; it is also known as tetrahydrocannabinol.
GW is focused on discovering, developing and commercialising novel therapeutics from its proprietary cannabinoid product platform in a broad range of disease areas.
Image: Sativex will be marketed in Switzerland by Almirall. Photo: courtesy of GW Pharmaceuticals plc.


