Eleison Pharmaceuticals received orphan drug designation from the European Commission (EC) for ILC (inhaled lipid-complexed gisplatin), for the treatment of osteosarcoma, a type of bone cancer.
The move follows the earlier positive opinion and recommendation of the European Medicines Agency (EMA) Committee of Orphan Medical Products.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The orphan drug designation grants the company access to protocol assistance and certain financial incentives from the EMA and also ten-years marketing exclusivity for ILC following the receipt of marketing approval.
Eleison Pharmaceuticals chief medical officer Forrest Anthony said ILC is potentially a breakthrough in the treatment of children and young adults with osteosarcoma, an often deadly cancer with little improvement in survival over the past 25 years.
"Our global phase II clinical for ILC remains ongoing with interim results expected in the middle of next year," Anthony said.
Eleison has an exclusive worldwide licence to ILC, which was designed to deliver high levels of sustained release cisplatin targeted to the lung, without systemic-related toxicities.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAdministered as an inhalational formulation of cisplatin, ILC is currently being evaluated in a Phase II clinical study at leading medical centres in the US.
Cisplatin is a platinum-based DNA synthesis inhibitor that targets DNA and cross links it causing inhibition of cell growth.
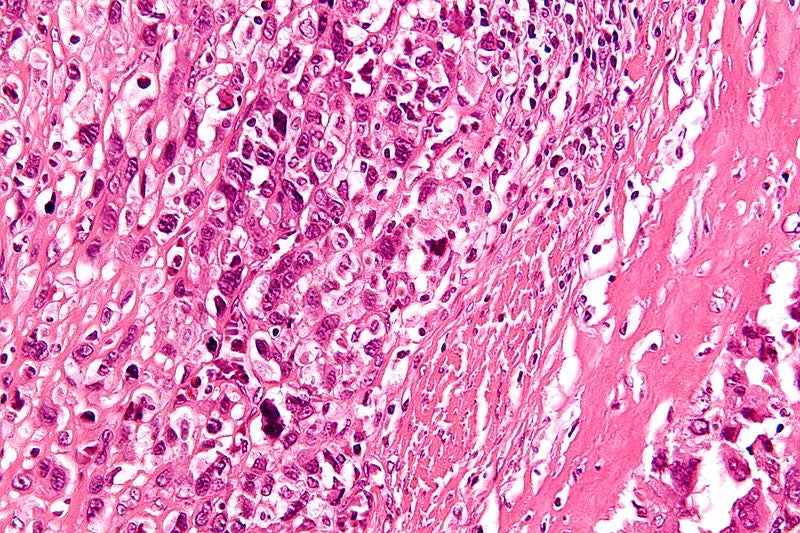
Image: A magnified micrograph of a high-grade osteosarcoma. Photo: courtesy of Nephron.




