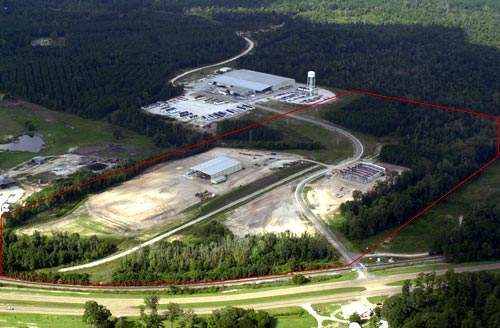
Stirling Pharma’s pharmaceutical plant in North Sydney, Nova Scotia, Canada, on a 300-acre business park was commissioned by Keata Pharma, a small contract manufacturing and packaging company, which was a subsidiary of the larger Korean concern, PharmaEng.
Keata wanted to capitalise on its unique ability to produce and package small runs of generic pharmaceuticals in solid and liquid forms. It had expertise in more complex production of small run pharmaceutical products and hoped to establish it as a niche business in this area of North America.
The company invested $15m in the construction and outfitting of the facility, and employed more than 150 personnel from the local area over a three-year period.
The plant has been constructed on a five-acre site in the Northside Industrial Park purchased from Cape Breton Regional Municipality, with a buying option for five years on an adjacent five-acre plot.
The plant opened officially in November 2007 and initially employed 65 personnel. Production began in July 2008.
and outfitting of the facility.”
The plant was closed in May 2009 owing to lack of supply contracts. It remained idle until March 2010, when it was acquired by Stirling Pharma for an estimated $3.6m.
Stirling Pharma revived the plant and brought it back into operation. In October 2010, Stirling Pharma obtained a site licence from Health Canada’s Minister of Health, permitting the manufacturing, packaging and labelling of natural health products at the facility.
On 10 January 2011, the facility was granted an establishment licence by Health Canada. The license was issued following a Good Manufacturing Practices (GMP) inspection concluded in December 2010. Manufacturing activities commenced on 19 April 2011. Production will increase gradually with additional contracts that are in the process of being signed.
Facility
The 4,270m² facility houses pilot laboratories dedicated for formulation development. There are suites equipped with a range of capabilities including high shear mixing, container blending and equipment for modified release technology. The plant will also include an R&D section at a later stage.
Production
The plant will offer formulation development and testing services, in addition to developing and packaging natural health products and over-the-counter drugs in solid and liquid dosage forms. The designed annual capacity of the plant is more than 550 million for tablets, five million for bottles and up to 1.5 million kilos for product blending.
Contractors
Keata Pharma was a subsidiary of a larger Korean-based full-service consulting pharmaceutical contractor and engineer, PharmEng Technology. During the construction period, PharmEng Technology acquired the necessary equipment and approvals to get the facility up and running and ready for FDA validation.
A local company, JoneLJim Concrete Construction, carried out the building construction. The parent company PharmEng expected the new facility to provide $50m worth of new manufacturing capacity for Keata Pharma.
Keata offered contract manufacturing services such as formulation development, pharmaceutical support and production and packaging of solid dosage and liquid products.
Financial assistance
PharmEng and Keata Pharma received financial assistance for setting up the new plant and establishing the new training course at the university.
Funds came from Enterprise Cape Breton Corporation (ECBC), the Cape Breton Growth Fund (CBGF) and Atlantic Canada Opportunities Agency (ACOA), as well as Nova Scotia Business Inc. (NSBI).
The ECBC and CBGF have invested $6.25m in the form of repayable long-term loans to assist with costs. ACOA paid $375,000 to assist with the $500,000 cost of setting up the training programme via their business development programme. NSBI provided $3,590,800 as a performance payroll rebate.
PharmEng also received $125,000 from the ECBC as a non-repayable contribution towards costs.
PharmEng Technology has also established a pharmaceutical consulting office in Cape Breton, employing an additional 12 professional staff.