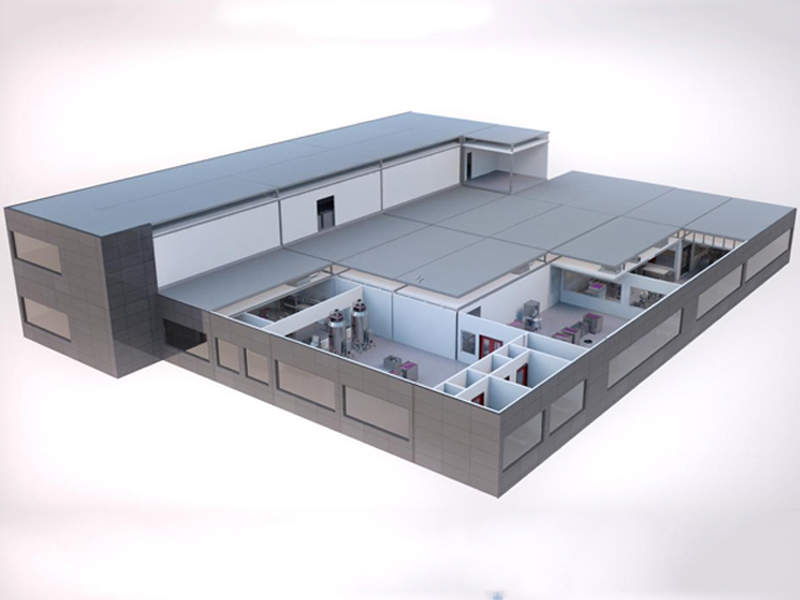
BeiGene Biologics, a joint venture (JV) of BeiGene Hong Kong and Guangzhou GET Technology Development, is developing a biologics manufacturing facility in Guangzhou in China’s Guangdong province.
The JV will develop the facility through its subsidiary, BeiGene Guangzhou Manufacturing, and provide financing for the research and development (R&D) of biologics in China, as well as developing the facility. The total investment in the new facility and R&D is expected to be CNY2.2bn ($330m).
Construction of the facility began in October 2017, and the first phase is expected to be completed and operational in 2019. The facility will promote high-quality, large-scale manufacturing to increase biologics production and meet the growing demand for BeiGene’s products in the Chinese and global markets.
Location of BeiGene’s biologics manufacturing facility
BeiGene’s biologics manufacturing facility will be located in Sino-Singapore Guangzhou Knowledge City (SSGKC) in GDD, Guangzhou. GDD is one of the initial 14 national economic and technological development zones approved by the state council in 1984.
The SSGKC is located roughly 35km from the Guangzhou city centre and 25km from Guangzhou Baiyun International Airport. It covers 123km² of land, with a start-up area of 6.27km². The SSGKC is aimed at attracting knowledge-based industries, including artificial intelligence, biotechnology and clean technology.
The BeiGene facility is expected to enhance the development of the biotechnology industry in the region while promoting its economic growth.
BeiGene biologics manufacturing facility details
The biologics facility will be a 24,000l commercial-scale facility developed on a 100,000m² site. It will use GE’s KuBio™ FlexFactory prefabricated manufacturing line to manufacture biologics.
The KuBio is a single-use configurable modular factory, which bolsters upstream and downstream bioprocessing efficiency at reduced costs. It delivers a prefabricated facility with a ready-to-use production line within 18 months, which is less than the traditional time period of around 24-36 months.
The prefabricated modules can be easily assembled at the site into a functional bioprocessing facility.
The facility will use a genetically modified cell line co-developed and licensed from Boehringer Ingelheim as the core raw material for manufacturing biologics.
Financing for the BeiGene biologics manufacturing facility
BeiGene Biologics will contribute CNY200m ($30m) for the development of the biologics manufacturing facility, while GET will contribute RMB1bn ($150m). GET’s contribution includes cash in equity investment of BeiGene Biologics and a shareholder loan, which may be converted into equity of the JV.
The company has also borrowed CNY1bn ($150m) from a commercial bank for the facility’s construction and operation.
The Guangzhou government also will provide support in the form of funding and providing the correct business environment for the facility’s development and operation.
Contractors involved with the BeiGene biologics manufacturing facility
BeiGene awarded the procurement and construction contract to Australian construction firm Cockram. Ireland-based consultant PM Group is responsible for providing site master planning and engineering design services for the facility.
PM Group is collaborating with EDRI, a local design institute, to complete the project.
General Electric was contracted to supply its state-of-the-art KuBio bio-manufacturing equipment for the facility.
Marketing commentary on BeiGene Biologics
BeiGene Biologics is a JV under an agreement signed in March 2017 between BeiGene Hong Kong (95%) and Guangzhou GET Technology Development (5%). BeiGene Hong Kong is a subsidiary of BeiGene, while Guangzhou GET Technology Development is an affiliate of Guangzhou Development District (GDD).