
Any metal contamination in pharmaceutical products is a huge problem. First and foremost, there are possible safety implications for patients that may ingest contaminates. For manufacturers, contamination leads to lost revenue and damaged customer relationships, as well as investigations, remediation, and in extreme cases, closure of manufacturing facilities, incurring huge losses.
In some scenarios, this can lead to supply disruptions, delays, inventory shortages, product recalls, regulatory scrutiny, and reimbursement for lost drug substances. Litigation can harm a company’s reputation for contaminant-free production, leading to decreased sales and additional costs.
To protect both the patient and the industry, pharmaceutical millers, a key step in the drug manufacturing process, must have robust measures in place to ensure no metal particles can get through to the final product.
Where does metal contamination come from?
Despite stringent quality control, microscopic metal contaminants that are in the sub-visible size range (<50μm) may go unnoticed. While several current techniques and analytical methods are available to detect, isolate, and identify such impurities, the chemical nature of metal corrosion makes it very difficult to identify the exact source of metal contaminants.
However, researchers have found that most metal contaminants in pharmaceutical products, such as tablets, are primarily caused by wear particles from processing machinery, such as stainless steel[i], and especially from machinery with moving parts like mills and tablet presses.
Supplier evaluation and control are crucial for ensuring quality standards, with raw materials being spot tested and inspected throughout the supply chain. Contract and owned manufacturing facilities must maintain the same standards and pass inspections to minimise contamination risks.
Additionally, customers of contract manufacturers frequently request metals testing as part of the supply contract and for regulatory submissions.
Strategies to prevent contamination
Pharmaceutical manufacturers and millers can prevent metal contamination in their products through various processes and quality control measures. These include:
- Manufacturing in approved facilities where specifications and standards ensure that the products meet regulatory requirements. The manufacturing process must be meticulously monitored to maintain quality standards and detect potential contamination, while performance and reliability testing is conducted to meet customer expectations.
- Conducting quality control testing of site laboratories as well as third-party labs. This ensures an independent verification of the product’s quality and helps in identifying any potential contamination issues.
- Implementing process controls to minimise the risk of contamination, such as metal detectors.
- Creating control points throughout the entire supply chain to help ensure compliance with the quality program and minimise the risk of contamination.
Detecting metal particles
To help address the issues of metal contamination, Frewitt, a premium mill and turn-key solution manufacturer, has developed an innovative metal detector that integrates with existing equipment to ensure continuous monitoring of powders.
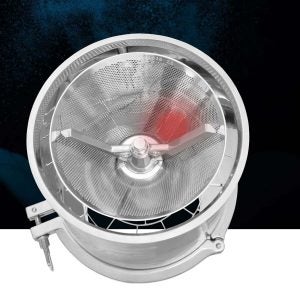
The Frewitt Metal Detector (FMD) sits inside the conical sieve and continuously sweeps the powder for any contacts. The FMD monitors the electrical isolation between the sieve and rotor, allowing machines to be immediately shut down in case of contact with a metal particle. The FMD detects any contact, including damage to the sieve surface, metallic foreign bodies, and even a missing sieve.
Available on Frewitt ConiWitt mills and sifters, the FMD ensures continuous measurement throughout the milling process, and is an ideal choice for removing the risk of contamination, process disruption and product loss. The FMD also helps validate FDA audits and can be retrofitted into existing machines, prolonging the lifespan of machines and tools by avoiding damage and wear.
Standard features include foreign metallic body recognition, sieve deformation recognition, automatic stopping, and full integration into the machine’s control system.
Given the serious risks and consequences that metal contamination in pharmaceutical products poses to patients, manufacturers, and the industry as a whole, Frewitt’s ground-breaking metal detector makes sure that drug manufacturers can always identify and remove these contaminants.
Based in Switzerland, Frewitt has been creating and producing high-end milling and handling solutions since 1946 while upholding the nation’s renowned quality standards. For more information, download the free paper below.
[i] https://www.contractpharma.com/issues/2010-10/view_features/metal-contamination-in-bio-pharmaceutical-drugs/


