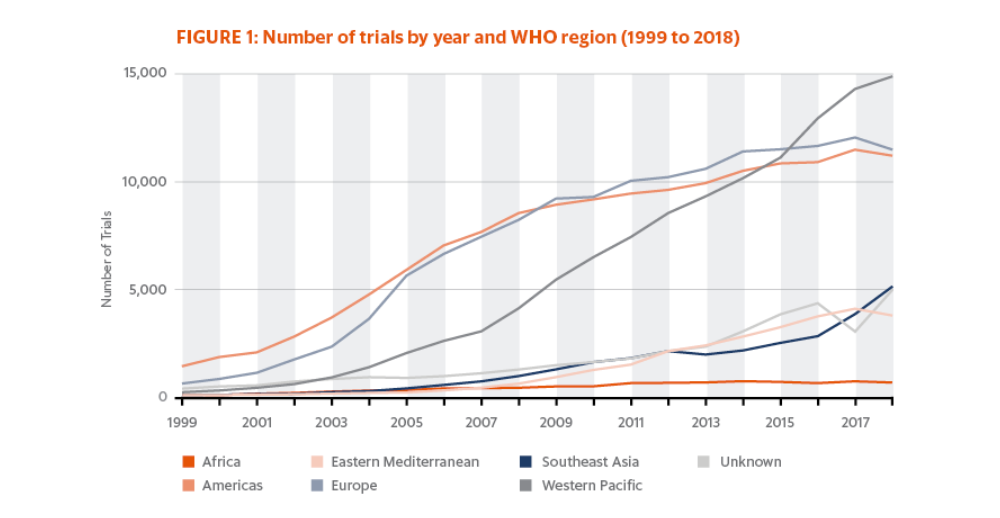
The World Health Organization (WHO) tracks clinical trials in the WHO International Clinical Trials Registry Platform (ICTRP) and has reported the number of trials by year, country, WHO region and income group for the period 1999–2018.1 The data includes both interventional and observational trials, with the year corresponding to the date of enrollment of the first trial participant.
Over this period, there has been a steady increase in the number of newly recruiting trials registered on ICTRP for most WHO regions (Fig. 1). By region in 2018 (counting multicountry trials conducted in the same region as one trial), the most trials in 2018 were initiated in the Western Pacific (14,655), followed closely by Europe (11,291) and the Americas (11,013). The fewest studies were begun in Africa (630), although there is growing interest in conducting trials in this region for drugs and vaccines designed to treat/prevent diseases native to the area, as well as efforts to expand the reach of international trials to include groups that have been historically underrepresented in clinical populations.
Since 2015, the number of trials conducted in the Western Pacific region has increased more rapidly than in other regions, and it is becoming the WHO region with the highest number of trial registrations per year. Most of these trials are taking place in Japan and China. This growth is in part due to the increase in requirements for sponsor companies to register trials in China to be able to gain access to that market. Not only are more clinical drug products being shipped into the country, but the shipment of patient laboratory samples has also increased dramatically.
Furthermore, trials registered in the ICTRP in 2018 originated in over 50 different countries. These trials involve all types of drug substances, from traditional small molecule drugs to highly complex biologics, from engineered proteins and antibodies to gene therapies and personalized medicines such as autologous CAR-T cell therapies.
In 2018 nearly 40% of drugs in the pharmaceutical industry pipeline were biologics, most of which require storage and handling at low temperature. In addition, a growing fraction of drugs submitted for approval are intended for the treatment of rare diseases with small patient populations (orphan drug or equivalent designation), leading to trials with fewer but widely scattered participants.
Reaching Distant Locations
Ensuring delivery of clinical trial materials and shipping biological samples to and from remote locations in a timely manner can pose significant challenges for clinical trial managers. Partnering with a clinical logistics provider that has an established, global network of depots, demonstrated knowledge of the regulations in these regions, centralized management and tracking systems and a highly trained workforce is often the key to success.
Some countries have complex and stringent regulations, the navigation of which requires detailed knowledge. In many developing countries, import and export regulations for drug products and biological samples are evolving rapidly. Having local staff that are in communication with regulators and customs agents is essential under these circumstances.
Through our global GMP-compliant depot network, which includes more than 21 different locations, we provide integrated services, including comprehensive transport of drug products and biologic samples, primary and secondary pharmaceutical packaging, warehousing and distribution support for cold-chain solutions, unused product return services and assistance with logistics project management.
The latter services include sourcing of comparator drugs and other supplies, establishing optimal delivery strategies across global trials and documentation support and the provision of regulatory advice regarding country-specific requirements. We also pay customs fees proactively so that shipments are never held up.
Our team of pharmaceutical transport specialists is highly experienced in supporting customer needs for the shipment of phase I, II, III, IV finished goods and production of raw materials. We guarantee speed of delivery with highly customized transport solutions and work with each client and every shipment on a one-to-one basis to ensure the highest level of service. Our goal is to ensure the fastest, most secure and most reliable delivery possible, anywhere in the world and with no size or weight restrictions.
Rapid Global Response
One dramatic example of Yourway’s global reach and agility was our involvement in delivering critical experimental drugs to patients during the 2018 Ebola outbreak in the Democratic Republic of the Congo. Ebola is a particularly deadly viral disease, killing about 50% of people who contract the virus.
In response to the growing outbreak, which numbered 58 cases in less than a month, and the limited available treatment options, the WHO and the Congolese government approved five experimental drugs — the monoclonal antibody cocktails ZMapp, REGN-EB3 and mAb114 and the antivirals favipiravir and remdesivir — to quickly address the unmet needs for the infected population.
Yourway was contracted by Regeneron to rapidly deliver REGN-EB3 from the United States to the Congo. We were able to leverage our existing distribution network to get this critical treatment to infected patients within 36 hours of the approval, playing a decisive role in helping contain the outbreak.
Managing Comparator Sourcing
To obtain drug approvals today, it is necessary to clearly demonstrate improved performance of a new drug product over existing therapies on the market. The best way to achieve this goal is to perform clinical trials with the candidate drug and a comparator, typically the commercial drug considered to be best-in-class at the time of the study.
Sourcing comparator drugs is rarely simple. In the best-case scenario, the sponsor company will have 12–18 months to organize the supply of comparator products. In practicality, the timeframe is much less, and in the worst-case scenario can be as little as a few weeks.
The complexity of the comparator sourcing problem varies with the drug itself and the countries in which the clinical trials is taking place. Many biologic drug manufacturers look to control the supply of their drug products and require supply agreements, even with sponsor companies looking for comparator material. Special licenses are required to ship comparator materials into some countries, while others require that drugs used in clinical trials be sourced from within the country. If the drug can be obtained at all, it may not be available in sufficient quantity, and multiple suppliers may be needed.
Yourway has developed strong relationships with many drug manufacturers and other key players in the pharmaceutical supply chain. We work diligently to establish supply agreements that will ensure access to sufficient quantities of comparator drugs when they are needed so as to avoid unnecessary delays, under-shipments and oversupply that can lead to the expiry of costly medicines.
Personalized Medicines and Trials
Personalized medicines — and cell and gene therapies in particular — often have very limited shelf lives and require storage and shipment under cryogenic conditions. Material is taken from a patient and shipped to the manufacturing site, where it is processed and formulated into a drug product that is then returned for administration to the patient. Yourway has the capability to ensure chain of identity and effective management of the cold chain combined with rapid shipment to and from locations around the world.
Yourway is also able to provide direct-to-patient shipment of clinical trial materials. Patients can experience the convenience of having their medicines delivered to their homes or workplaces, while clients can be assured that the medicines will be maintained under compliant environmental conditions. We can also develop customized delivery solutions if patients will be traveling or moving to a new location, making it possible to keep all patients enrolled and receiving their medications.
Changes Only Beginning
The pharmaceutical industry is evolving rapidly. As the understanding of disease mechanisms expands, pharmaceutical scientists are developing entirely new ways of curing patients. The types of drugs and formulations being developed today — biologics, patient-specific therapies and other specialized treatments — did not exist just 10–20 years ago.
There are also many more companies — largely small and emerging biotech firms — developing those novel medications. Finding enough patients to fully enroll the rapidly growing number of decentralized, international clinical trials is a challenge.
Innovation in drug discovery and development is driving the need for innovation in logistics technologies. For sponsor companies, efficiently and effectively managing the supply of the specialty clinical materials and patient samples used in and generated by the trials of today — and those in the future — requires partnering with a clinical logistics company committed to continuous improvement and the ongoing development of new packaging, sourcing and other logistics solutions.
References
“Monitoring processes to R&D.“ World Health Organization. Apr. 2019. Web.
Lloyd, Ian and Alexandra Shimming. “Pharma R&D Annual Review 2018,” Pharma Intelligence presentation. 16 May 2018. Web.