
Drug shortages worldwide are worsening due to increased demand for high-quality manufacturing capacity in the pharmaceutical industry. Short-term contingency plans from healthcare providers and governments can help, but long-term solutions are needed to increase quality and capacity, reduce downtime, and minimise product wastage.[i]
Continuous line processing is more efficient than batch processing and can free up both capacity and human resources, with faster, high-quality, processing of drug products, helping alleviate the global drug shortage.
However, one pain point is characterisation in dry granulation systems. This method is restricted to offline processes, as reliable, continuous, inline monitoring has not yet been commercially viable. Up until now, product was removed from the line, tested, measured, and then wasted.
How an inline, real-time PAT tool characterises granules
While roller compactors create granules from powder, a measurement method is needed to characterise the granules, which are responsible for the behaviour on a tablet press. The most important parameters are the particle size distribution of the granules and the density of the ribbon, which affects particle distribution and flowability.
At gap density, the density of the ribbon in the moment of maximum compression between the press rollers, can be calculated dividing mass flow by volume flow. Volume flow can be calculated using the press rollers dimensions, speed and gap. Mass flow can be measured by a scale. However, the existing method is not suitable for continuous production lines due to the use of an IBC (Intermediate Bulk Container) positioned on a platform scale. Further, this method requires a large measurement range and high accuracy of the platform scale to be able to measure compared to the system’s weight small mass flow.
“Our customers have been asking for better ways to monitor granule properties inline, but until now, nobody has found a really good solution,” says Michael Schupp, Head of Process Engineering at Gerteis, a specialist in complex granulation processes.
“We wanted to solve two problems here,” explains Schupp. “One is to be continuous, and the other to remove the need for an expensive platform scale.”
Launching at ACHEMA 2024, Gerteis has leveraged its expertise to design a brand-new PAT tool to measure inline ribbon density, allowing for closed-loop feedback and with automatic press force adjustment, able to maintain the defined density range during production. Importantly, this process does not stop the continuous line.
The Gerteis Inline Density Control
“The solution is here,” says Schupp. “We created our own weight and density measurement system, the inline density measurement, as an add-on for machines of the Gerteis Pactor® series.”
Schupp explains how the inline system works:
“We have two valves in the outlet product stream of the roller compactor, the upper valve and the lower valve. The lower valve is positioned on weighing cells. We use the upper valve to control the product flow and the lower valve for the measurement.”
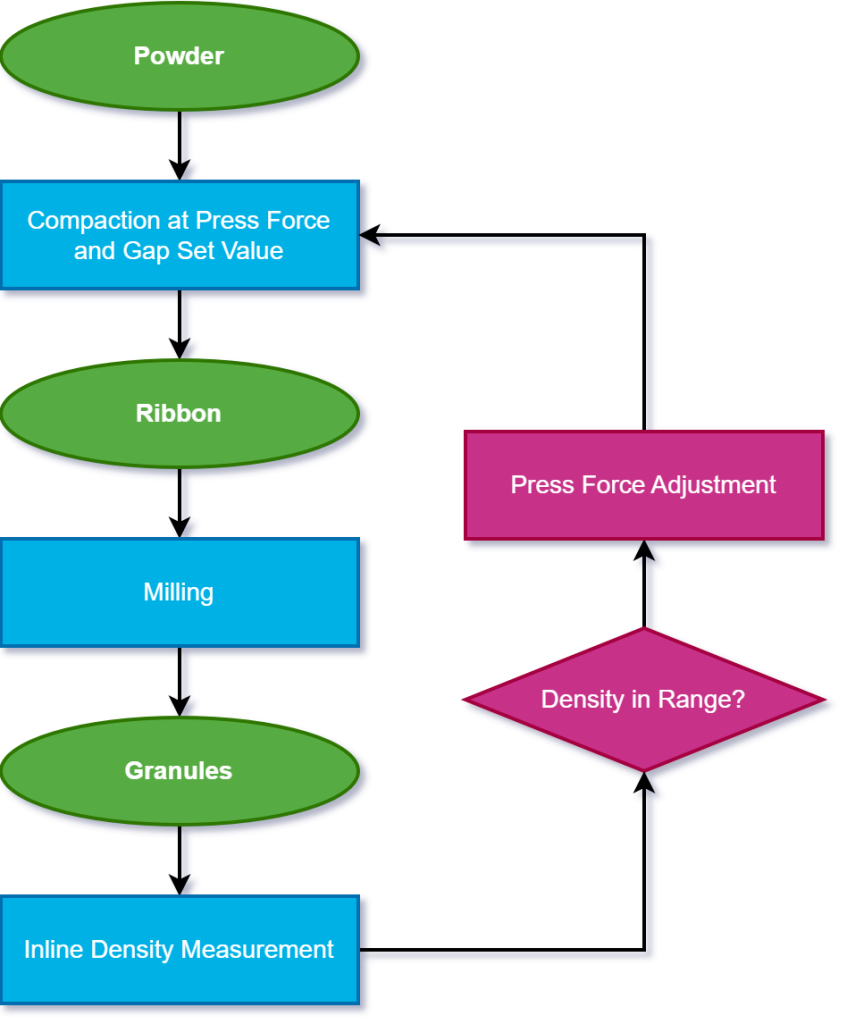
The inline density control system also automatically monitors and adjusts press force to achieve the desired density.
“Every time we have a new measurement, the inline density control system is going to adjust the press force if required,” says Schupp.
The software includes settings for density, measuring time, correction limits, and press force adjustments. It is easy to operate, supports PAT and QbD and does not require a product-specific calibration.
Ultra Small Amount Funnel
Alongside the new PAT tool, Gerteis is also revealing its latest add-on for machines of the Mini-Pactor® series, the Ultra Small Amount Funnel. Ideal for laboratory development and pilot projects, this brand-new option can process materials with as little as 10 grams of product. This is a huge improvement for those customers that only have very small amounts of product available.
Additionally, almost no product or residuals remain in the equipment, making it an excellent ‘high yield, small amount’ option, with very little wastage.
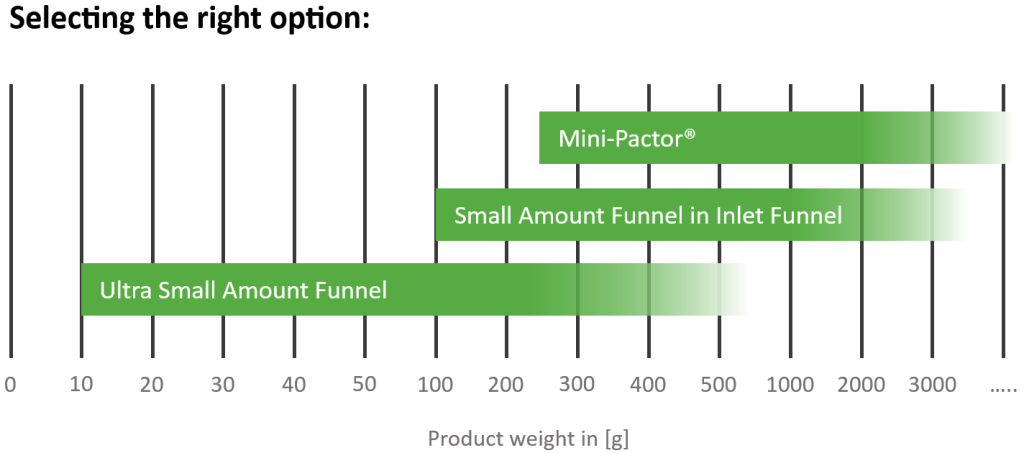
The funnel seamlessly integrates with the compaction unit of the Gerteis Mini-Pactor® and is designed to provide the same superior performance you would find in a full-scale production machine, ensuring all results are accurate and scalable.
More efficient, less waste
Schupp believes the industry can improve efficiency, increase capacity, and reduce drug product wastage through these new products, alongside other Gerteis innovations.
“You need fewer human resources, which allows you to run more equipment with the same number of operators at a higher quality,” he says. “With these tools, you’ll be able to control quality and also be able to have a direct release process. That’s something many pharmaceutical companies are working on at the moment. They want to have reliable PAT tools to be able to release the product immediately – getting the product to market quicker.”
For more information on how Gerteis can help with your granulation process, visit www.gerteis.com
[i] GlobalData: New Drug Approvals and Their Contract Manufacture: 2024 Edition (page 53)


