
Recent trends highlight a surge in demand for mass market medicines. This increase is driven by lessons from the Covid-19 pandemic, when vaccines had to be made available quickly in large quantities, but also from the ongoing need for diabetes or weight loss medication. GMP-compliance, quality control, risk of contamination as well as cost of production and time to market are some of the increasingly significant challenges that companies delivering large batch sizes are confronted by. Having a competent and efficient partner by your side, capable of addressing these challenges through top-notch integrated end-to-end solutions can elevate your mass production to new levels of efficiency and sustainable success. Here’s where the unique Körber Ecosystem approach comes into play – ensuring worldwide healthcare from the initial idea to the finished solution: with everything, effortlessly, in one place.
Highlights:
- What are the biggest challenges of pharma mass production?
- How to streamline your mass production and reduce complexity by leveraging the Körber Ecosystem approach?
- Which integrated end-to-end solutions does Körber offer?
In the dynamic landscape of the pharmaceutical industry, companies are continually grappling with opposing trends. On the one hand, there’s a growing demand for individualized medicine and small batch sizes tailored to specific patient groups. On the other hand, the ageing of the world’s population and the increase in obesity, for example, have led to a greater need for corresponding mass-market medication worldwide.
While some pharmaceutical companies are specialized in individualized medicine and therefore need to produce small, flexible batches, others focus on the large-scale production of mass market medicine. These companies must be able to scale their production to produce large volumes of products without compromising on efficiency, quality, and safety. Regulatory compliance then becomes more complex, and the risk of contamination or errors can increase. Furthermore, the cost of production can escalate quickly if not managed effectively. Time-to-market pressures necessitate rapid development and commercialization of new drugs and any delays in production can result in lost market share and revenue. Solving these pain points holistically enables fast production which can support the need for big batches. This is where we step in.
How to streamline your pharmaceutical mass production and reduce complexity by leveraging the Körber Ecosystem
With several decades of experience in the pharmaceutical and biopharmaceutical industry, cutting-edge technologies and a worldwide sales & service network, we are well-equipped to navigate the complexities of your mass production – taking weight off your shoulders and ensuring worldwide healthcare by doing so.

We offer integrated end-to-end solutions which aim to refine, optimize, and streamline your mass production processes. Our unique Ecosystem approach addresses all areas critical to large-scale production – from regulatory compliance, risk of contamination, quality control, cost of production and time-to-market pressures.
Regulatory compliance
With stringent regulatory requirements across large batch sizes, maintaining compliance can be challenging. Here, Körber’s powerful and market-leading PAS-X MES Suite comes into play, digitalizing and automating documentation and reporting tasks, ensuring each of your batches adheres to Good Manufacturing Practice (GMP) standards.
Risk of contamination
Guaranteeing sterility and preventing cross-contamination are critical in pharmaceutical manufacturing. Any deviations might lead to product recalls, regulatory sanctions, and damage to public health and trust which then lead to economic losses. And as the scale of your production increases, so does the risk of contamination. By minimizing human interventions in production processes through automation, robotics and closed manufacturing systems such as Line Optimizer, the risk of contamination can be drastically reduced. Accurate equipment performance can be ensured with the right aseptic processing solutions and thus reduce downtime while maintaining optimal cleanroom conditions.
Quality control
Ensuring consistent quality across your mass production batches is a complex task due to variations in raw materials, equipment performance, and environmental conditions. Körber’s cutting-edge inspection machines uses AI to significantly reduce reject rates, which leads to increased throughput within production as there are fewer false rejects, enabling proactive quality control measures across all batches. Software-supported process management, e.g. with PAS-X Savvy and PAS-X MES, enables the early detection of deviations, and compliance with regulatory standards at all times.
Cost of production
As your production scales up, managing costs becomes more intricate. As there are a lot of factors influencing your Overall Equipment Efficiency, it can become increasingly challenging for you to keep key figures at a high level.
Körber’s consulting services optimize production processes, reduce labor costs, and minimize errors, thereby lowering the overall cost of production and additionally reduce the footprint within a line, making it more sustainable.
Time-to-market pressures
Accelerating product development and commercialization is crucial for companies involved in mass production. Körber’s machinery business, in terms of handling, inspection, packaging, materials, and supply chain solutions, speed up these processes by leveraging digital manufacturing technologies and continuous manufacturing systems.
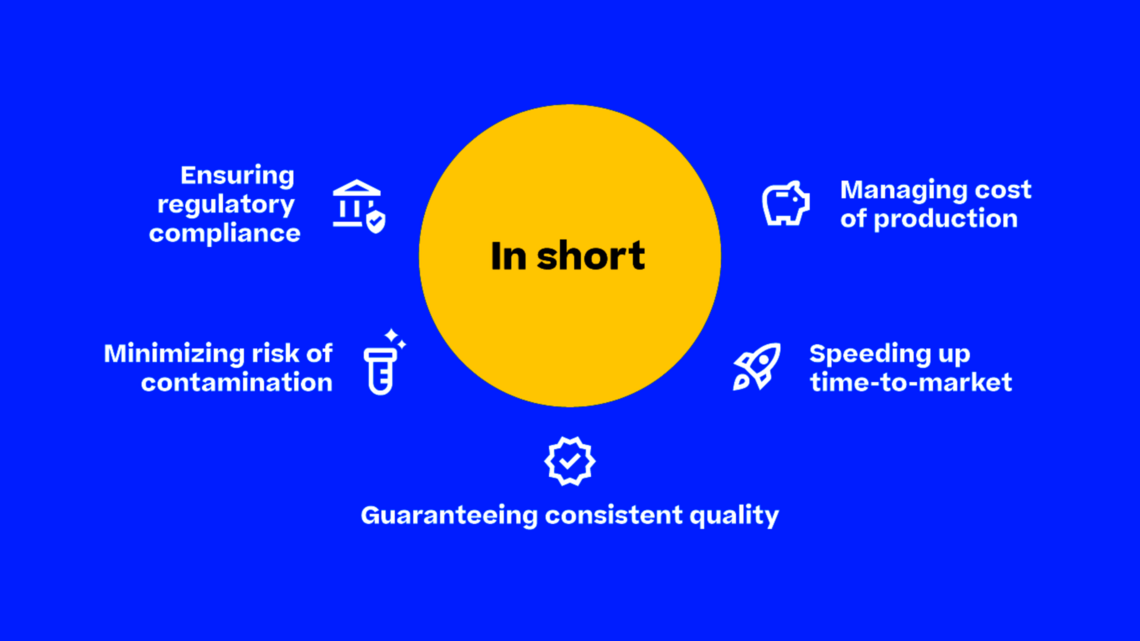
By integrating various components of the Körber Ecosystem, its interconnectivity empowers you to effectively navigate the complexities of mass production in the pharmaceutical industry. This synergy allows for a holistic approach to tackling your individual pharma challenges. No matter what the specific needs of your company, as long-time experts in the pharmaceutical industry we provide tailored solutions including innovative machines, cutting-edge software, top-tier services, and digital solutions.
From the first idea to the finished product, we provide solutions along the entire value chain. Whether you’re looking to optimize your supply chain, enhance operational efficiency, increase sustainability or ensure regulatory compliance, we are your reliable and strategic partner towards mass production excellence.
Why you should choose us
Our track record and customer feedback speak volumes about our capabilities. Explore these case studies and blog articles and see for yourself:
- Small causes, big effects: 30 percent OEE increases through comprehensive production analysis
- It’s so much more than Electronic Batch Recording!
- 5 benefits of using a MES in pharma & biotech
Contact us today and let us support you in unlocking your pharmaceutical production potential, effortlessly and effectively. With our ecosystem, success is just a consultation away.
Looking for more information? Visit our website for comprehensive insights into our products and services.
If you have any questions or need further assistance, we are here to help. Use our contact form, or get in touch with one of our internal contacts:
Robert Köck
Körber Business Area Pharma
Head of Digital Marketing & Demand Creation
+49 175 3009 939
robert.koeck@koerber.com
We look forward to hearing from you.
Contact us today


