
The pharmaceutical industry faces myriad challenges affecting its supply chain, from stringent regulatory requirements to the urgent need for agility and digitalisation. Logistics service providers – LSPs – can help to smooth out common kinks in the pharma supply chain both by overcoming these challenges and harnessing opportunities to innovate and improve efficiency.
A significant barrier to agility and innovation in the pharma sector is the reliance on legacy systems. While regulatory compliance is non-negotiable, the rigidity of old systems may stifle transformation, so forward-thinking LSPs must marry compliance with modern technology. They can provide cutting-edge technology, act as a strategic ally in achieving supply chain optimisation, ensure compliance and enhance competitiveness in the global market by staying ahead of emerging trends.
The impact of artificial intelligence and big data on pharma logistics
Artificial Intelligence (AI) stands at the forefront of transformative technologies in the pharma supply chain, with applications ranging from eliminating inefficiencies to predictive analytics. AI’s capacity to analyse vast data sets can significantly improve demand forecasting, anticipate supply chain disruptions, and even refine patient trial processes through synthesised data.
Utilising AI and machine learning means that the pharmaceutical industry is no stranger to big data. The right LSP recognises that data management involves more than just storage and analysis; it requires a strategic approach to security, privacy, and utilisation. By implementing robust data security measures and employing advanced analytics, these providers transform data from a potential liability into a powerful tool for optimisation.
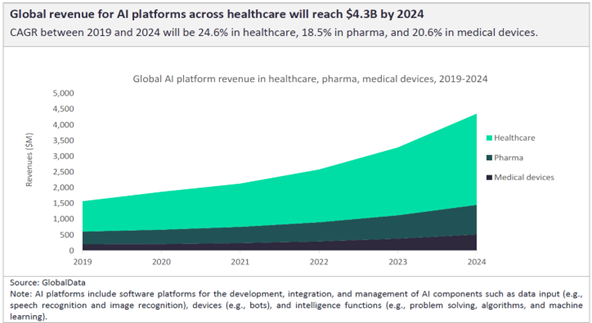
Embracing this digital transformation in the pharmaceutical supply chain is vital. The right LSP plays a crucial role in this process, offering solutions that streamline the transition to digital operations while ensuring compliance with industry regulations. Leading pharma firms select the providers that offer platforms and technologies designed to enhance visibility, improve efficiency, and facilitate real-time decision-making, making them invaluable in the quest for digital maturity.
A range of software solutions are hitting the market to help LSPs maximise efficiency. Platforms such as Controlant’s Aurora Platform exemplify how tailored, cloud-based technologies can revolutionise data visibility, offering real-time insights into every stage of the supply chain—from shipments to monitoring device health—facilitating a seamless transition to digitalisation with minimal disruption.
Regulatory expertise and collaboration
The pharmaceutical industry’s stringent regulatory landscape presents unique challenges, especially when it comes to logistics and supply chain management. LSP partners of pharma companies need to work in tandem with regulatory bodies, leveraging their expertise to accelerate the adoption of new technologies and practices. In doing so, they help pharma companies maintain compliance while exploring new avenues for efficiency and growth.
LSPs using Controlant’s real-time IoT loggers and Aurora Platform also benefit from Controlant’s wealth of expertise and experience in pharmaceutical supply chain management – invaluable for navigating the complex regulatory landscape, enhancing operational efficiency and reducing risks associated with product integrity and compliance. Controlant’s solutions, characterised by their scalability and flexibility, can help LSPs meet the needs of pharmaceutical companies, allowing them to adapt to changing market demands and regulatory environments.
By providing detailed insights into the status and location of products throughout the supply chain, LSPs can help pharmaceutical companies manage risks effectively, comply with audits and make informed strategic decisions. Additionally, the platform’s ability to collect and analyse vast amounts of data enables LSPs to go the extra mile, helping their clients uncover opportunities for improvement and reduce operational costs.
The impact of industry trends
In the pharma industry today, procurement leaders are under pressure to rethink sourcing strategies to accommodate the shift towards customised medicines. Traditional Requests for Proposal (RFPs) and sourcing processes often fall short in addressing the needs of complex supply chains. As customised medicines come to the fore, so too will technologies such as Controlant’s platform, offering scalable and tailored solutions that address the unique challenges of the pharma supply chain. By focusing on strategic procurement, talent management, and fostering partnerships with suppliers, LSPs can work with pharma companies to navigate supply chain complexities and ensure the efficient delivery of life-saving medicines to those in need.
Unit-level tracking marks a significant evolution in pharma logistics, shifting the focus from bulk shipments to the granular tracking of individual units and unlocking new opportunities for efficiency. For example, by employing digital twins for scenario planning, companies can anticipate and mitigate potential disruptions before they occur. This level of detail transforms logistics from a static, historical record into a dynamic, predictive tool, enabling smarter decisions about distribution strategies, inventory placement, and route optimisation.
Controlant’s Aurora Platform is a transformative solution designed to enhance pharmaceutical supply chains through the lens of real-time monitoring, compliance assurance and unparalleled visibility. And, as the pharmaceutical industry continues to evolve, utilising LSPs who can see its supply chain through this lens will become increasingly important. Aurora ensures the integrity of pharmaceutical products from production to delivery by maintaining them within the required temperature ranges, thus safeguarding their efficacy. It also facilitates adherence to global pharmaceutical regulations, minimising the risk of non-compliance and ensuring products meet the highest standards of quality and safety. Whatever the future of pharma supply chains looks like, this platform is helping LSPs to adapt – and their pharma clients are sure to benefit.
To find out more about how Controlant’s Aurora Platform could help you, download the whitepaper below.


