
Autoinjectors have revolutionised the convenient delivery of low-volume parenteral drugs. Traditionally, autoinjector combination products have been limited to injection volumes of 0.1 to 1.0 mL, with the world’s first 2.0 mL fill autoinjector having been commercialized just a few years ago. By shifting injections from clinics to living rooms, these single-use, ready-to-use devices have improved the patient experience and supported treatment adherence for an array of common chronic diseases. However, patients with conditions requiring higher doses, such as cancer, have yet to enjoy these benefits. For them, intravenous infusion in a hospital setting is the only option.
But with the advent of injection site absorption enhancers, drug delivery device innovations, as well as the recent primary container options, the practical subcutaneous self-administration of large-volume parenterals is becoming possible. This is an exciting prospect: oncology, and immuno-therapy in particular, is a driving force behind biotech R&D, with new cancer therapies and molecules accounting for nearly 40%1 of the industry’s development pipeline.
The question now is how to best design and manufacture autoinjectors that can be used to safely store and administer these larger volumes.
The challenge with prefilled syringes
Most autoinjectors have fill volumes of 1.0 mL or less and use a prefilled syringe (PFS) with a staked needle as the drug’s primary container. In recent years, some PFS autoinjectors with larger injection volumes have been launched, such as SHL Medical’s Molly 2.25 autoinjector.2
Treating atopic dermatitis, high cholesterol, and other chronic conditions, these devices have demonstrated that larger-volume subcutaneous injection is feasible in a home setting. Increasing their volumes further, however, may be more challenging.
Yet PFSs also come with a challenge such as presenting difficulties in maintaining the stability of protein-based molecules or complex monoclonal antibodies. These stability issues arise in PFSs in the form of leachables due to the presence of silicone oil3 and tungsten residue4 from the manufacturing process, as well as from other contact materials like the adhesive used to bond the needle and barrel.
Protein aggregation and particle formation5 can occur due to silicone oil-water and air-water interfaces, making the siliconisation of the primary container crucial. While baking the silicone can reduce silicone migration and protein aggregation6, this process is challenging and poorly suited for PFSs: it requires temperatures exceeding 300°C, compromising the bond between the staked needle and the glass barrel.
The case for cartridge-based solutions
A promising alternative is the adoption of cartridges, as highlighted by SHL Medical’s Maggie® autoinjector.7
A trusted primary container within the pharmaceutical industry, cartridges have several properties suited to sensitive biologics, including limited contact materials and the absence of tungsten. The lack of a staked needle also lends itself to high-temperature baking to reduce protein aggregation and particle formation.
However, cartridges alone still offer limited accessibility. Conventional cartridge-based solutions are complex and require users to manually attach the needle to the device, risking contamination and injuries.
The Maggie autoinjector addresses this limitation by incorporating a cannula unit featuring SHL Medical’s Needle Isolation Technology (NIT®). The NIT unit consists of a sterile self-contained cannula with patient and non-patient ends. As the needle cap is removed, the non-patient end moves backwards to pierce the cartridge septum and open the drug fluid path (Figure 1). The patient end of the needle remains hidden at all times by a sliding cover, which also activates the autoinjector when pushed against the injection site.
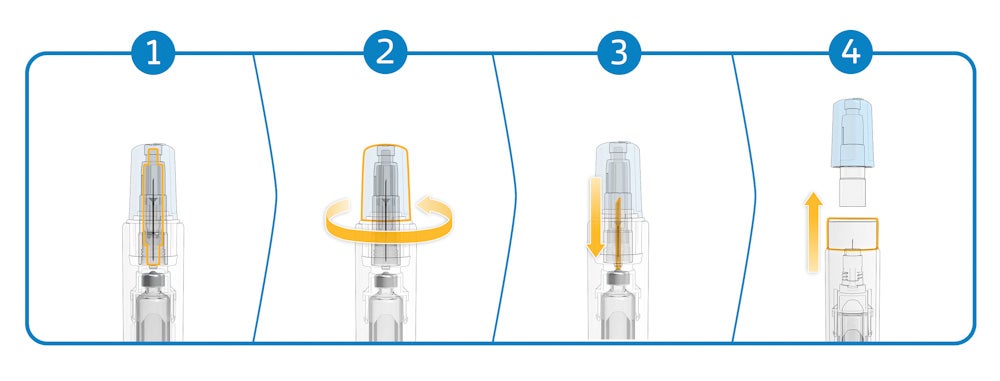
The NIT thus simplifies the injection process by eliminating the need for patients to attach the needle to the cartridge, making for a simplified and safe end-user experience – and freeing autoinjectors to carry larger doses than existing PFS-based devices.
3.0 mL, 5.0 mL, and beyond
Combining the advantages of an easy-to-use, cartridge-based autoinjector with large-volume subcutaneous injections, the Maggie 3.0 mL autoinjector debuted in 2017. And, with various studies continuing to show8 that even larger doses may be administered subcutaneously, the Maggie 5.0 mL autoinjector9 was introduced in 2022, increasing fill volume further.
The 5-mL device is aimed at supporting therapy areas requiring higher injection volumes, including in the oncology market. Its compact design and optimised dimensions mean that it is not significantly larger than other successful, lower-volume SHL Medical autoinjectors (Figure 2).

Large-volume, high-dose subcutaneous autoinjections are soon becoming a reality. SHL Medical knows that widening the use of autoinjectors for volumes of up to 5.0 mL and beyond requires deeper technical investigations to better understand the influence of injection variables such as time, drug volume and viscosity, as well as injection depth. Through its Maggie family of autoinjectors, SHL is working with its partners to advance the device development side of combination products, together gaining key competitive edges in this burgeoning subcutaneous drug delivery market.
To learn more, download the document below.
References
1. Citeline Pharma Intelligence report – Pharma R&D Annual Review 2022: Navivating the Landscape
2. SHL Medical’s Molly 2.25 Autoinjector
3. National Library of Medicine – Silicone Oil Induced Aggregation of Proteins
4. National Library of Medicine – Precipitation of a Monoclonal Antibody by Soluble Tungsten
7. SHL Medical’s Maggie Autoinjector
9. SHL Medical’s Maggie 5.0 Autoinjector


