
Despite the best efforts of pharmaceutical manufacturers and regulators to improve the security and traceability of drug products, counterfeits continue to proliferate and cause considerable harm both to patient outcomes and to profitability. To tackle this growing threat, as discussed in the first article of this series, the pharmaceutical industry must explore innovative measures, such as on-dose authentication, which promises more direct and foolproof methods of assuring drug authenticity.
In October 2011, the FDA issued “Guidance for Industry: Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form (SODF) Drug Products for Anticounterfeiting.” According to the guidance, a physical-chemical identifier (PCID) is defined as “adding a trace amount of an inactive ingredient to authenticate legitimate [drugs], and to identify counterfeits.” Examples of PCIDs include inks, pigments, flavors, and molecular taggants.
According to the FDA, PCIDs should be pharmacologically inert, ensuring they do not affect the drug’s safety or efficacy, and can be considered pharmaceutical excipients. The placement of PCIDs is crucial for ensuring their safety and effectiveness. Integrating them into the core of SODF drugs could lead to interactions with the API, potentially causing degradation of both the drug product and the tracer. Therefore, placing the PCID in an external component, such as the coating of a pill or tablet, is recommended to mitigate this risk as is using the minimum level necessary for clear identification. Additionally, the FDA recommended pharmaceutical manufacturers use direct food additives that are generally recognized as safe (GRAS) or listed in the FDA’s Inactive Ingredient Guide (IIG).
PCIDs that meet these criteria can be incorporated into already approved drugs as a Level 1 post-approval change, offering the simplest pathway to implementation. Unfortunately, these criteria are quite limiting. Other PCIDs, including SECURtracers, require that a pharmaceutical manufacturer include the PCID in a new drug product submitted under an NDA or ANDA. Once the FDA has approved such a use, the FDA agency will consider including the new tracers in the IIG, making the post-approval route possible for following users.
On-dose authentication facilitates the identification of counterfeit drug products by absence of the tracer. Unlike traditional packaging-based security measures, on-dose authentication provides a direct method of verification that remains with the product itself.

SECURtracers can be integrated into the coating of a drug, the matrix of pills or tablets, or the shells and band seals of hard or soft capsules.
Standard SECURtracers are sized at 100x200x10 microns, with a count of four million particles per gram. Each particle contains ten or more micro-engraved letters or symbols. Both the dimensions and microengraving of SECURtracers are fully customizable to meet customer requirements.
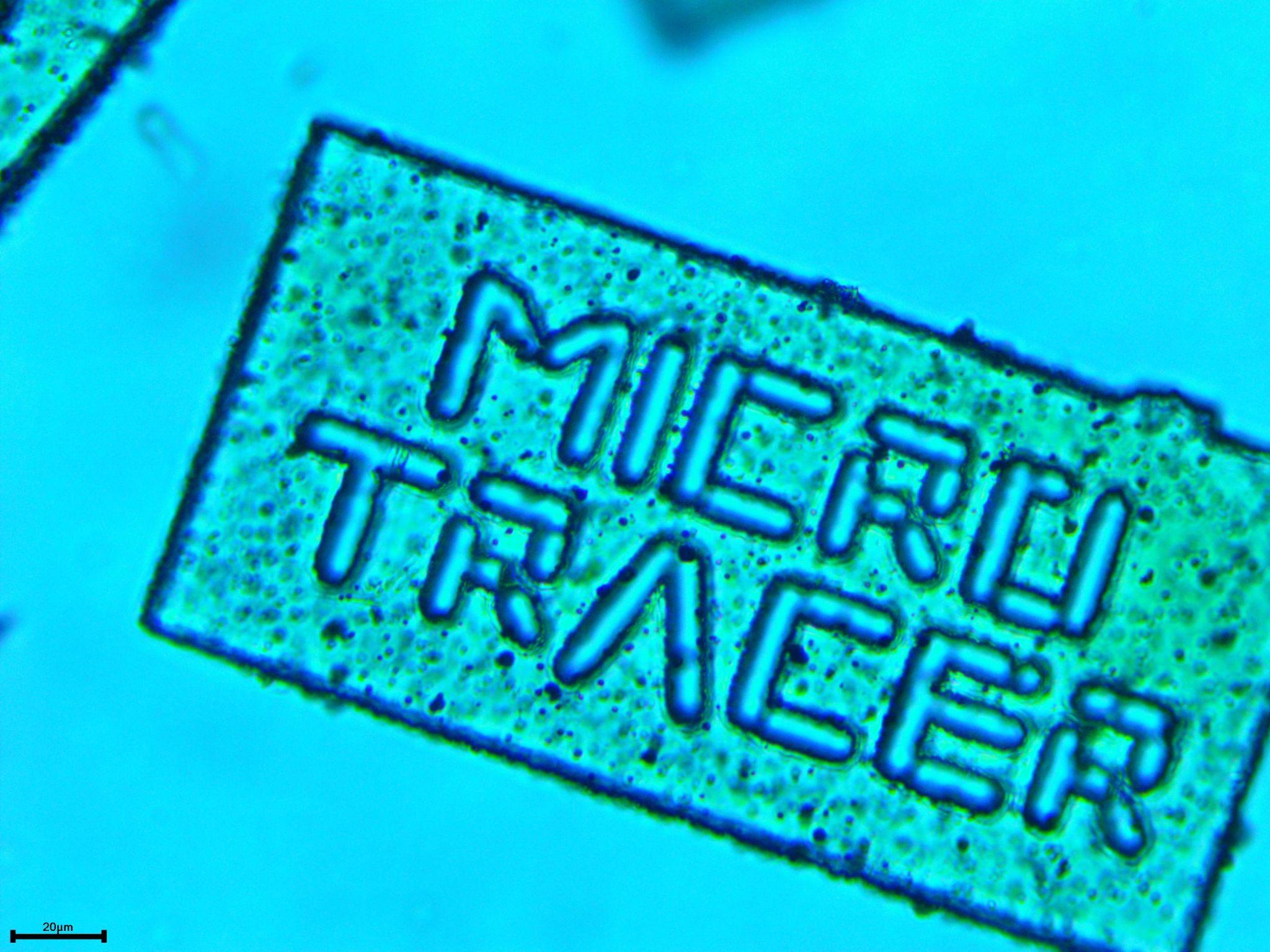
SECURtracer particles are composed of GRAS materials: an ethyl cellulose USP/NF grade matrix embedded with curcumin derived from turmeric oleoresin and carbonyl iron powder. The ethyl cellulose serves as the “matrix scaffolding” of the tracer, while the functions of the curcumin and carbonyl iron powder are critical to allow SECURtracer identification, thereby facilitating rapid verification of drug authenticity.
Specifically, when SECURtracers are integrated into the drug coating, curcumin exhibits bright fluorescence under UV black light. This feature enables a quick preliminary test: scanning the drug product’s surface with a UV black light reveals yellow-gold fluorescent specks. The absence of these specks signals a counterfeit product. Notably, in normal lighting, the SECURtracers are invisible, leaving the drug’s appearance unchanged.

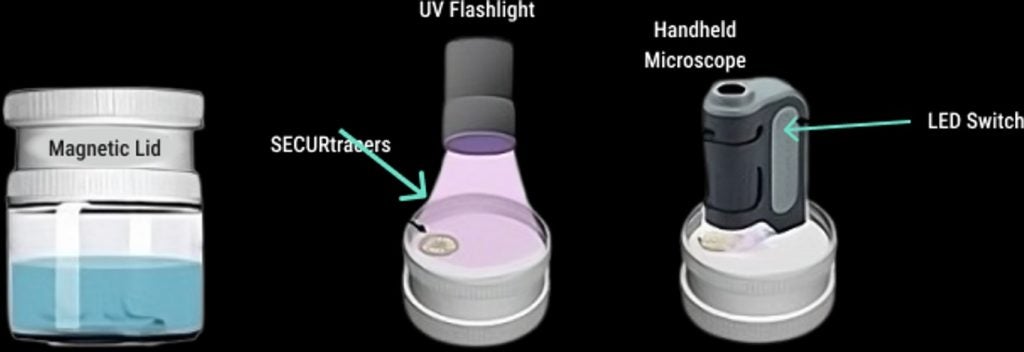
The carbonyl iron powder makes the tracers magnetically retrievable. When the drug product is dissolved in water, the tracer particles can be isolated on a rare earth magnet in the lid of a small plastic jar, then examined as seen in the graphics below. Using a 60-120X hand-held microscope, the micro-engraved letters and symbols on each SECURtracer can be read and determined to be a match with the tracer formulated in the specific drug product, confirming its authenticity.
Typically, SECURtracers are added to solid oral drug products at a concentration of 12.5 to 25 ppm (12.5 to 25 grams per metric ton of drug product). Extensive testing has shown no detectable impurities found with a maximum level of detection (LOD) of 250 ppm. If the SECURtracer is formulated at 12.5ppm in the drug product, this equates to a maximum for any identified impurity of 3 ppb in the drug product. Both the US FDA and the European Medicines Agency (EMA) have advised such low level impurities (as a maximum) are not significant. This SECURtracer formulation is adequate to assure the presence of tracers, even if mixing is not complete. For more cost-effective solutions, smaller SECURtracers measuring 50x50x10 microns and containing just two micro-engraved characters can be used at concentrations as low as 2.5 ppm, still adhering to FDA-PCID guidelines and minimizing costs.
The FDA’s proactive recognition of the need for PCIDs highlights the critical role on-dose technology will play in ensuring drug traceability and authenticity. PCIDs are complementary to existing measures such as track and trace and packaging solutions. Unit-level security and traceability systems can be maintained, with pill-level authentication introduced for high-value or high-risk drugs.
Since 1961, Micro-Tracers has successfully manufactured similar magnetic-based tracers for the rapid identification of drug products in animal feeds, with over 2 billion tons formulated without a single reported adverse reaction. Leveraging decades of its parent company’s expertise, SECURtracers are positioned to facilitate this paradigm shift in human drug authentication.


