Takeda Pharmaceuticals opened a vaccine manufacturing plant in Singen, Germany, in November 2019, for the production of its dengue vaccine candidate, TAK-003.
The plant will perform end-to-end production and packaging of TAK-003, which is being investigated in a pivotal phase three trial, Tetravalent Immunization against Dengue Efficacy Study (TIDES).
The ground-breaking ceremony of the plant was held in 2016. Takeda will invest more than €130m ($145m) in the state-of-the-art facility, which will create more than 200 jobs.
The facility is expected to expand Takeda’s global footprint in vaccine manufacturing outside Japan, while allowing the company to combat the universal threat of dengue.
Dengue is considered an endemic in more than 120 countries and is one of the fastest spreading mosquito-borne viral diseases. The World Health Organization (WHO) estimates that approximately 390 million dengue infections and 20,000 deaths occur a year worldwide.
Takeda’s dengue vaccine manufacturing plant location and details
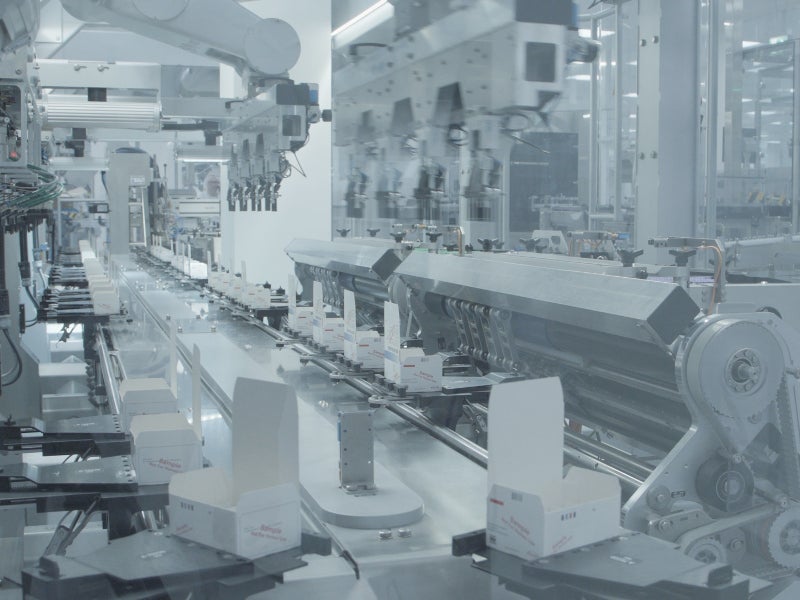
Singen was selected as the strategic location for Takeda’s dengue vaccine manufacturing plant due to the availability of an expert workforce in lyophilisation technology, crucial in the production of TAK-003 vaccine.
The production plant will perform formulation, fill-and-finish and packaging of the TAK-003 vaccine. It employs a high degree of automation and state-of-the-art digital and data-driven technologies.
The data-driven manufacturing environment allows for real-time monitoring of the plant’s performance, providing superior quality assurance delivered to the patients at the final stage.
Takeda’s TAK-003 dengue vaccine details
TAK-003 is a tetravalent dengue vaccine developed by Takeda. It is a live-attenuated dengue serotype two virus (DENV-2), the genetic backbone for all four vaccine virus stereotypes including DEN-1, DEN-2, DEN-3 and DEN-4. The drug has demonstrated its effectiveness in generating an immune response in phase one and two trials and was safe and well-tolerated.
The multi-centred, global, double-blind and randomised placebo-controlled TIDES trial is being conducted to evaluate the safety, efficacy and immunogenicity of TAK-003 in healthy children living in the dengue-endemic countries of Latin America and Asia. More than 20,000 healthy children aged between four and 16 have been enrolled for the clinical study.
Initial results from the trial, announced in January 2019, showed that the experiment met the primary endpoint. The drug was effective in preventing dengue caused by any of the four virus stereotypes. Additional data from the clinical trial will be published towards the end of 2019, along with data from separate phase three clinical trials. The vaccine is currently not approved anywhere in the world.
TAK-003 vaccine is being developed to protect children and adults living in or travelling to endemic areas against all four stereotypes of dengue viruses, regardless of previous dengue exposure.
Marketing commentary on Takeda Pharmaceuticals
Takeda Pharmaceuticals is a global biopharmaceutical company, which focuses on innovative research and development. Founded in 1781, the company is headquartered in Tokyo, Japan.
Takeda’s research is mainly centred around four therapeutic areas including oncology, neuroscience, rare diseases and gastroenterology (GI). The company also invests in research to develop plasma-derived therapies and vaccines.
Takeda has a distinguished product portfolio, as well as several investigational drugs in the pipeline. Current products being offered by the company include Nesina (Alogliptin benzoate), Velcade (Bortezomib), Adcetris (Brentuximab vedotin), Colcrys (Colchicine) and Dexilant (Dexlansoprazole).
Alongside the TAK-003 dengue vaccine, Takeda is also developing vaccines for Zika, norovirus and polio.