SAFC Pharma’s St Louis facility is a cGMP biologics manufacturing facility, which undertakes plant and animal-based therapeutic protein extraction and purification, bio-conjugation as well as excipients and adjuvants manufacturing.
A new high-potency active pharmaceutical ingredient (HPAPI) conjugates facility was opened at the site in 2008.
SAFC Pharma cGMP facilities
The facility comprises separate cGMP manufacturing, extraction and purification units for animal-sourced APIs, plant-sourced APIs, bio-organic APIs synthesis and purification, and potent and non-potent conjugation.
The cGMP manufacturing unit includes a pre-weight room, a main reactor room, a mezzanine reactor room, a crystalliser room, a mezzanine large filter room, a main large filter room and a small filter-cum-shelf dryer room. Raw materials are brought to the pre-weight room. The air-locked room is equipped with vessels for transferring the raw material through slurring.
Designed in compliance with process safety management guidelines, the cGMP manufacturing unit is custom-made to handle flammable solvents in large quantities. It provides total segregation and enclosed processing, and uses United States Pharmacopeia (USP) purified water, process control and temperature-controlled HEPA-filtered air.
The facility’s main reactor room is a bio-organic reactor suite equipped with glass-lined jacketed vessels with capacities of 750 gallons, 200 gallons and 100 gallons. The suite is installed with fully validated equipment and clean-in-place (CIP) systems.
The crystalliser room has a glass-lined crystalliser with a capacity up to 3,000 gallons. A 50-gallon receiver and condenser is devoted to the crystalliser. The filter rooms with side discharge areas allow products to be packaged without environmental exposure. The small filter-cum-shelf dryer room is equipped with 0.6m2 filter jacketed vessels used for heating and cooling. Product separation is done via a peeler centrifuge and/or through large vacuum filter dryers up to 2m2 in size.
The new cGMP HPAPI conjugation suite is spread over 800ft2 of floor space. The class 100,000 (ISO 8) suite is equipped with dual-sided, six-glove isolator and reactors ranging between 10l-100l in size. It has a batch size capacity of more than 100g.
Proteins derived from natural plant sources are purified in large, 1,054ft2 ISO 8 and 660ft2 Class 10,000 (ISO 7) suites. Animal-based proteins are purified in large, 940ft2 ISO 8 and 600ft2 ISO 7 suites. The suites are equipped with 1.5m-diameter chromatography columns.
The plant-based extraction and purification unit can process up to 5,000kg of plant-based biomass and 6.5kg of therapeutic protein every year. It has a 225ft2 lyophilisation room with a capacity of 200l.
The extraction room is installed with three 2,500l extraction tanks and one 7,000l buffer tank. The clarification rooms have one 3,200l tank and two 2,000l tanks.
Production for biologics
The SAFC Pharma St Louis facility provides biologic APIs and conjugation technologies from the preclinical to commercialisation stage.
The plant produces animal-sourced and plant-sourced therapeutic proteins that are used for the development of key biologic drugs.
Customised and high value biologic products are also developed at the facility for use by the biopharmaceutical industry as raw materials, excipients or adjuvants.
Adjuvants manufactured at the facility include keyhole limpet haemocyanin (KLH), polyarginine, and polyinosinic acid / polycytidylic acid that are respectively used for cancer vaccines, treatment of several infectious diseases and for multiple vaccines including autoimmune cancer and infectious diseases.
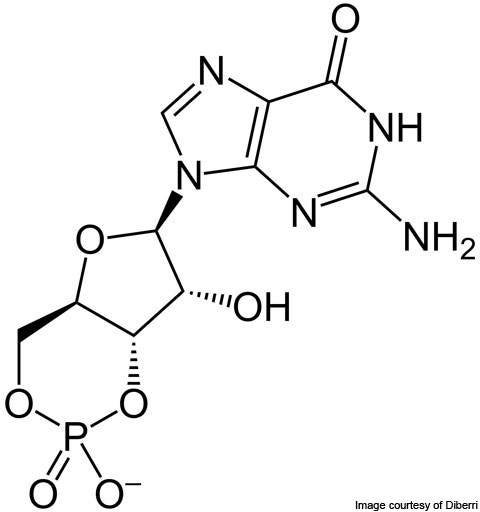
The SAFC facility offers bio-organic synthesis and purification of bio-organic intermediates, excipients and bulk actives as well as conjugated HPAPIs that are used to develop new oncology products. HPAPIs are conjugated with biological molecules.