pSivida is an Australian nanotech company specialising in the commercialisation of biodegradable nanotech silicon devices. They are used for the administration of isotopes to tumour cells and also the monitoring of treatment regimens in patients. However, the biodegradable drug delivery technology is also useful for the administration of a wide range of other drugs. One application has been the administration of the tanning medication Melanotan, involving Epitan and pSivida.
The company’s main focus is the development and commercialisation of a modified form of silicon (porosified or nano-structured silicon) known as BioSilicon. BioSilicon offers multiple potential applications including controlled-release drug delivery, wound healing, targeted cancer therapies (such as brachytherapy and localised chemotherapy), tissue engineering and orthopaedics. Potential diagnostics applications are being developed through a subsidiary, AION Diagnostics Limited.
PSivida’s strategic partner and largest shareholder is the QinetiQ group, the former UK government Defence Evaluation Research Agency (DERA), which was instrumental in discovering the porous medium, which is BioSilicon.
MANUFACTURING PLANT IN BRAUNCHSWEIG
pSivida’s manufacturing partner QSA completed the construction and validation of a state-of-the-art cleanroom facility in September 2005. Part of a three-year manufacturing agreement signed in March 2004, this was dedicated to the supply of pSivida’s lead cancer therapy BrachySil, at the Auriga radiopharmacy facility in Braunschweig, Germany.
The QSA facility carries out the final stage in the manufacturing process for future clinical and commercial use. Auriga Medical manufactures BrachySil from BioSilicon particles using a process of neutron bombardment. Auriga Medical also manages the supply logistics for clinical and commercial use.
During the initial development phase Auriga Medical optimised the manufacturing process of BrachySil for both clinical trial and commercial supply. The facility operates under cGMP manufacturing guidelines and is dedicated to the supply of BrachySil.
MANUFACTURING PLANT IN SHEFFIELD
The UK manufacturing partner, Atomising Systems Ltd, also reached a key milestone in the manufacture of BrachySil. The construction of a dedicated cleanroom facility to GMP specifications at its Sheffield plant in the UK was completed in 2004. The new facility now enables pSivida to increase BrachySil production in support of two large clinical trials planned for later in 2006. Advanced liver cancer trials will take place at Singapore General Hospital and five other Asian centres and new Phase IIa trials in pancreatic cancer will occur in Singapore and London.
For future commercialisation, pSivida used the Sheffield facility to produce ultra-pure nano-structured BioSilicon microparticles doped with phosphorus. These microparticles were created using a specially developed melting and water atomisation process. The production of ultra-pure silicon alloy powder involved melting and atomisation with high pressure water sprays at speeds of 200m/sec to produce particles in the 10–100μm range.
Both plants play a large role in the manufacturing process. Atomising Systems produces the microparticles of BioSilicon doped with phosphorus and QSA carries out the final neutron bombardment activation stage of the process. All key steps and facilities involved in the BrachySil manufacturing process are now operating to the essential GMP requirements. These are necessary for regulatory approval, which was granted toward the third quarter of 2006. pSivida raised £20m for the development of Brachysil, including the construction of the new cleanroom facilities at Sheffield.
BRACHYSIL
BrachySil (32-P BioSilicon) was developed by pSivida for the direct intratumoural treatment of cancers. BrachySil has shown excellent results in Phase IIa clinical trials as a radiotherapy for the treatment of inoperable primary liver cancer. It is delivered directly into the tumours without surgery, in a procedure known as brachytherapy.
Clinicians receive BrachySil as a sterile powder for resuspension in an injectable aqueous formulation. This is then administered via a fine gauge needle, using ultrasound or tomography for accurate placement. The brachytherapy market is currently worth over $600m per annum and is expected to exceed $1bn within the next few years.
BRACHYSIL REPORT FOR PANCREATIC CANCER
In January 2008, the pSivida BrachySil in Pancreatic Cancer Study Results were presented at the ASCO Gastrointestinal Cancers Symposium. The study was a Phase IIa trial where 17 patients were treated with BrachySil delivered directly to a tumour in the pancreas via endoscopic ultrasound – used to assist in locating the delivery point – in combination with standard chemotherapy.
Pancreatic cancer actually has one of the lowest cancer survival rates, a five-year relative survival rate of approximately 4%. In addition, 85–90% of patients are diagnosed with the inoperable form of the disease.
There is significant clinical and market demand for effective therapies to treat this aggressive form of cancer, which is the fourth leading cause of death by cancer in the US and Canada. The continuing trial will now monitor the efficacy of the treatment based on CT measurements of tumour regression as a foundation for the subsequent Phase IIb dose-profiling studies.
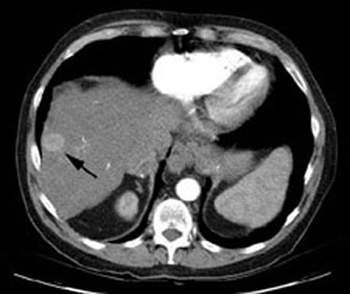
The system has also been trialled in liver tumours (first indication). A Phase IIa clinical study on BrachySil in patients with inoperable hepatocellular carcinoma (HCC) (five-year survival rate of 7%) was carried out using a single fixed dose (4MBq per ml tumour). The dose was administered intratumourally using the silicon device to demonstrate the safety and initial efficacy of the product.