Emergent BioSolutions launched a new biotechnology manufacturing facility on 16 July 2010. Formerly known as the MdBio Bio Processing Centre, the facility is located in Baltimore, Maryland and was acquired from MdBio Foundation in November 2009 for $8.2m.
It was constructed in 1996 and has operated under several ownerships. Unyil 2000, the facility was owned by Bio Science Contract Production (BSCP) Corp, a New York-based contract manufacturing organisation.
In 2000, the facility was acquired by generics manufacturer Cambrex that operated the facility until Lonza bought it in 2007. Lonza operated the facility briefly until Mdbio took it over following Lonza’s decision to prune its capacity.
The facility will begin production by early 2012. During this course of time, Emergent BioSolutions plans to renovate and re-outfit the plant at an approximate cost of $30m.
Approximately $15m is expected to be spent on the renovation by the end of 2010.
The renovation will enable Emergent BioSolutions to expand beyond the bio-defence sector.
It will also allow the company to pursue more government work besides penetrating deeper into the commercial sector.
The new facility will create up to 125 jobs within a span of three to five years.
Facility
The new facility includes 56,000ft² of manufacturing and administration space. Nearly 11,000ft² of space has been dedicated towards manufacturing activities. There are five separate manufacturing suites that have been designed specifically to support clinical and commercial production of Emergent BioSolution’s product pipeline which includes rPA, anthrax monoclonal and tuberculosis drugs.
The facility additionally accommodates five laboratories, sterile hallways and white walled workrooms. It is equipped with stainless steel bioreactors and can support concurrent manufacturing.
The company’s remodelling plans will combine and convert the five laboratories in the facility into two. This will allow Emergent to develop viral and non-viral vaccines. To expand the footprint of the site, a plot lying adjacent might also be acquired by the company.
Production
The facility will be used to produce Biothrax, a sterile, pure-white suspension that is the only anthrax vaccine approved by the FDA. Emergent has obtained several large government contracts to manufacture and stockpile Biothrax.
In addition, the facility will test potential viral and non-viral vaccines for other common diseases including tuberculosis and typhoid. The development pipeline of Emergent BioSolutions also includes drugs targeting botulism, hepatitis B and chlamydia.
Technology
The facility will use disposable manufacturing technology to develop vaccines. The technology requires lesser capital investment and is expected to fast-track the process development at a lower cost of operation.
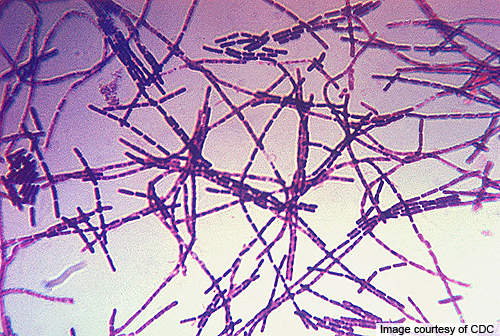
Biothrax will be produced from cell-free microaerophilic culture filtrates of a non-virulent, non-encapsulated strain of the anthrax causing microbe, Bacillus anthracis.
Production cultures will be harvested in a chemically controlled protein-free medium that will include a combination of acids, vitamins, inorganic salts and sugars.
The final product developed through the sterile filtrate culture fluid will comprise proteins including 83kDa protective antigen protein that is formed during the growth phase.
The product does not contain bacteria in any form, dead or alive, and is composed of 1.2mg/mL aluminium that is added as aluminium hydroxide in 0.85% sodium chloride.
Finance
A $50,000 grant was secured from the Maryland Department of Business and Economic Development to conduct assessment studies for the facility, as part of the Brownfields Revitalization Incentive Program. The program is aimed at promoting economic growth.
A $250,000 credit on the cost of the site was offered by Baltimore city (that owns the site) in order to boost job creation. Additional Job Creation Tax Credits offered to the facility include the One Maryland Tax Credit. The facility can also enjoy other benefits of being located in an enterprise area.