The Danish biotech company Bavarian Nordic has set up a new smallpox vaccine production facility in Kvistgård, North Zealand, Denmark. The new facility has been set up to supply increased demand for smallpox vaccine around the world, following an increased awareness of the possibility of bioterrorism. Thirty different governments worldwide have placed orders with the company or made serious enquiries.
Bavarian Nordic has acquired a production facility from the Finnish company Orion Pharma. The 9,000m² facility is situated 30 miles north of Copenhagen on a 26,000m² site. Bavarian Nordic has also made this location its administrative centre as well as its main production facility.
The facility was completely refitted with brand new plant, equipment and facilities and validated by both the FDA and EMEA as a production facility. The total investment has amounted to DKK250m (€33.6m, $41m).
The plant was ready for its refit by mid 2004 and the work was completed in less than eight months, allowing test production (validation series) to be completed by the third quarter of 2005. The plant was then in full production by the final quarter of 2005, with the ability to produce 120 million doses per year of the new smallpox vaccine. The initial workforce was 50 employees. The filling, inspection, labelling and packaging/finishing of the sterile vaccines has been placed with Impfstoffwerk Dessau-Tornau (IDT), a partner of Bavarian Nordic with its own modern facility in Germany.
Contractor and refitting
Novo Nordisk Engineering (NNE) was given the contract to construct the new production facilities inside the acquired building. The production plant is now used for the production of a smallpox vaccine and possibly vaccines against other infectious diseases should the need arise. NNE also assisted Bavarian Nordic with the preparation of regulatory approvals for an assessment of the production facility’s impact on the environment (constructed according to biosafety level-2 standards).
The facility includes 1,100m² of Class B and C cleanrooms, tank farm storage facilities, two bioreactors (500l and 1,200l volumes) and the associated downstream processing facilities, along with steam on-line for clean in place (CIP) activities, and sterile water and water for injection for cleaning and producing batches of media for culture respectively. The vaccine production is also fully current Good Manufacturing Practice (cGMP) compliant and adheres to the current biotechnology safety rules for containment of the production process to protect the environment.
NNE was contracted to carry out a cleaning validation in case of a contamination of production from previous activities at the plant. This was required as part of the cGMP compliance validation. In addition, NNE was asked to reconstruct an administration block and 400m² of laboratory buildings for the extensive QC/QA activities of the new vaccine plant. The QC laboratory was granted authorisation and release permissions on production batches of IMVAMUNE by the Danish Medicines Agency (Lægemiddelstyrelsen) in mid-2005.
Bavarian Nordic vaccine
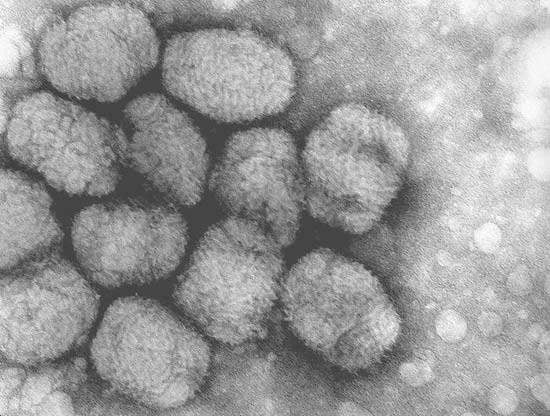
In the search for a vaccine against HIV Bavarian Nordic came across a new type of MVA vaccine against smallpox, which it has tested and produced for three years. The Modified Vaccinia Ankara (MVA-BN) is Bavarian Nordic’s lead vaccine vector and is based on an attenuated poxvirus (classified as a biosafety level-1 vaccine).
Recombinant MVA-BN vaccines have a number of important characteristics including a high expression of antigens in target cells. They are also potent inducers of Cytotoxic T Lymphocyte (CTL) and antibody responses and have a high efficacy in humans. The MVA-BN vaccines are safer than other Vaccinia viruses since they are highly attenuated and do not replicate in mammalian cells. The new MVA-BN vaccine is well tolerated in immune compromised patients, is serum-free, has a high titre production and may be stored for extended periods by freeze drying at 4°C.
The new MVA-BN vaccine is being marketed under the name IMAVMUNE and is currently in Phase II clinical trials, along with another similar vaccine based upon the MVA-BN vector to combat HIV. The smallpox vaccine was granted an Investigational New Drug (IND) designation in May 2004 by the FDA and this means that the drug is available for sale to governments such as the US, Germany, UK and Greece. It was not available for general release until late 2005. Prior to the new plant coming on-line IDT manufactured the virus for Bavarian Nordic in Germany.
New safer vaccine
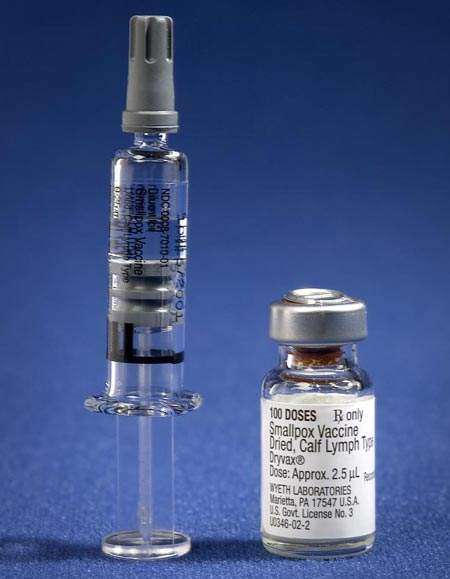
Clinical studies have compared traditional Vaccinia smallpox vaccines, such as Dryax manufactured by Wyeth-Ayerst, with the Bavarian Nordic MVA-BN third generation smallpox vaccine IMAVMUNE and have found there are far fewer side effects. The new vaccine is safe to be given to patients in known high-risk groups such as immune compromised, elderly, pregnant and young patients, along with those suffering from atopic dermatitis. The previous vaccines were known to elicit a very severe response in, or be dangerous to, these high-risk patients.
The US government is seriously considering using this ‘safer’ vaccine for the entire nation.
Bavarian Nordic was awarded a contract from the US Department of Health and Human Services in May 2005 for 80 million doses (RFP-III draft contract). The plant can produce 60 million doses per year.
The US NIH also ordered a further 500,000 doses of the vaccine under an earlier RFP-II contract for delivery in 2006 for research and evaluation, produced under cGMP conditions from the new facility. The RFP-II contract period for evaluation was extended to 2009–10 to include clinical studies with subjects having atopic dermatitis, a large risk group.
In November 2009, the (Biomedical Advanced Research and Development Authority (BARDA) placed a $40m contract with Bavarian Nordic for freeze-dried version of the IMVAMUNE smallpox vaccine. The contract value was increased to $94m in April 2011. The company will also supply 20 million doses of the vaccine in its current liquid-frozen form under the RFP-III contract. Denmark and a few other Nato countries placed orders for IMVAMUNE in June 2011.
The company plans to collaborate with commercial partners for late stage development of its clinical products and for international marketing. The company has worldwide partners including Chiron and Epimmune (US), Gerolymatos (Greece), IDT (Germany) and Vaccine Solutions of Australia.