Aphena Pharma Solutions expanded its solid dose division in Cookeville, Tennessee, US, with a $20m expansion and renovation, adding capacity for US Food and Drug Administration (FDA)-compliant pharmaceutical packaging of solid dose and biologic products.
The project expands the company’s operations into biologics, cold chain storage and third-party logistics distribution with future headquarters at the facility.
Aphena’s expansion of the solid dose and liquids divisions in Maryland will help it to become a diversified manufacturing and packaging company in the pharmaceutical industry.
The expansion makes the company a strategic growth partner for generic and over-the-counter (OTC) pharmaceutical companies.
Aphena’s solid dose division expansion
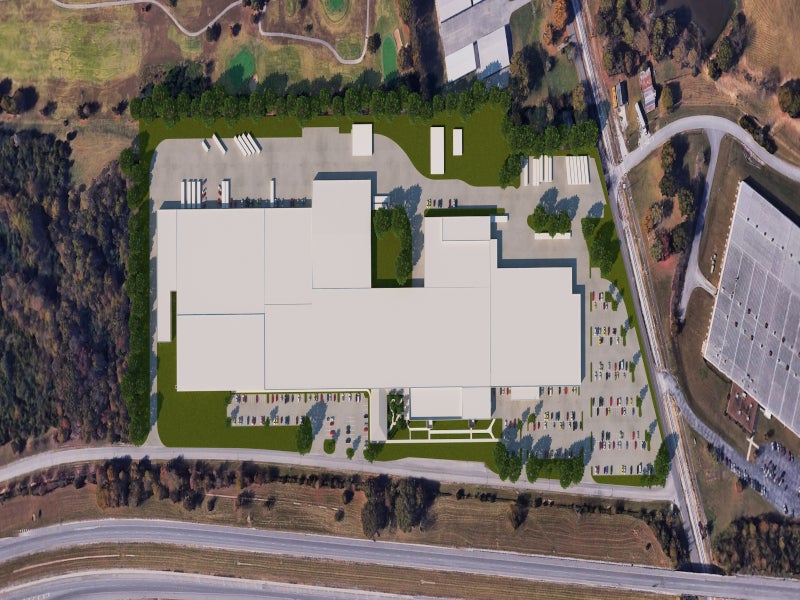
Aphena has expanded its solid dose division by adding an acquired 500,000ft² property for manufacturing and packaging operations registered with the FDA.
The facility was renovated into a state-of-the-art pharmaceutical packaging and distribution facility, which added 258,000ft² to the company’s existing 578,000ft² of manufacturing space in Cookeville. It expanded the company into biologics, cold chain storage and third-party logistics distribution.
The new facility features 200,000ft² of warehouse space to support third-party logistics programmes for existing and future clients, as well as 47,000ft² of space for cold chain storage with a capacity of more than 12,000 pallets.
Aphena added a high-speed solid-dose bottling line in February 2020, dedicated to packaging solid-dose OTC and prescription products in branded and private label categories. The line increases the facility’s capacity by approximately 15 million to 20 million bottles a year, allowing the company to maintain a 50% capacity level for future projects or accelerate capacity requirements.
The expansion has enabled Aphena to enhance its bottle packaging capabilities significantly. It initially introduced ten state-of-the-art high-speed bottling lines for solid-based items such as tablets, capsules, caplets, soft gels, and gel caps in January 2021.
The company plans to further install 30 more high-speed lines within the new facility. It will increase the monthly capacity to more than 80 million bottles. The expansion also allowed the installation of eight thermoform blister packaging lines.
Details of Aphena’s solid dose division in Tennessee
Aphena’s solid and semi-solid dose packaging facilities in Tennessee cover 578,000ft² and offer various packaging services, including bottle filling (glass and plastic), vial labelling and serialisation, cartoning, blister packaging (cold-form and thermoform blisters), pouching or bagging, secondary or serialisation packaging, PDQ displays, heat-seal or stretch-film cards, rework projects, and kitting.
The division provides packaging solutions for solid doses, including tablets, capsules, and soft gels. The solutions are assisted by comprehensive quality processes, project managers, customer service agents, and continuous development programmes.
Additional services offered by Aphena’s solid dose division include graphic support, clinical services, stability packaging services, package design, materials recommendations, child-resistant packaging support, and trade and physician samples.
Aphena offers its third-party logistics (3PL) services at the facility, with a focus on the generic segment, along with the OTC, pharmaceutical market, and nutraceutical/homoeopathic markets.
Aphena’s 3PL division details
Aphena launched its 3PL division in Cookeville in August 2021. The company offers 3PL logistics services, including full distribution services, order-to-cash services, controlled room temperature storage, US Drug Enforcement Administration (DEA) schedule II (vault) and DEA schedule III-V storage, and cold chain storage at temperatures between 2°C and 8°C.
Marketing commentary on Aphena Pharma Solutions
Aphena Pharma Solutions offers a range of pharmaceutical packaging and manufacturing services for the pharmaceutical, medical device and biological markets. In addition to the solid dose division in Tennessee, the company has a liquids and topicals division in Easton, Maryland.
Expansion of the Maryland facility involves the installation of two additional pharmaceutical blending suites with separate air handling systems, and two state-of-the-art tri-mix blending tanks for handling small and large batches of speciality creams, gels, and topical products.
The facilities cover a total area of 762,000ft² and offer 24-hour and six-days-a-week operations to provide industry-leading turnaround services and product launch capabilities. Both facilities are FDA and DEA-registered and are compliant with current good manufacturing practices (cGMP), ISO 13485: 2016, 9001: 2015, UL Certified, and USP verified, meeting various standards.
Aphena’s contract packaging and manufacturing solutions aim to allow its customers to launch their products in the market on time and on budget.