Alpha Biologics, a contract biomanufacturing service provider, commissioned a new cGMP biomanufacturing plant in April 2010. The plant, located at the Penang Biotech Park in Malaysia is dedicated to the production of mammalian cell secreted proteins.
The plant broke ground in June 2006 and was built at an investment of $18m between 2007 and 2009. It was constructed in compliance with the FDA and EMEA guidelines.
The design incorporates a flexible layout that was reviewed by the Centre of Drug Evaluation and Research (CDER) at the FDA and declared suitable for clinical studies and early market product development.
The facility has multiple rooms and functions that enable parallel working processes, thus reducing timelines and the eventual cost to the customer.
In the first quarter of 2010, Alpha Biologics was acquired by Viropro, a research and manufacturing service contractor based in Nevada. The acquisition was completed on 22 February 2010 for a consideration of $21m.
The facility is due to obtain accreditation under the Pharmaceutical Inspection Cooperation Scheme. The accreditation process is expected to commence in the next few months.
Facility
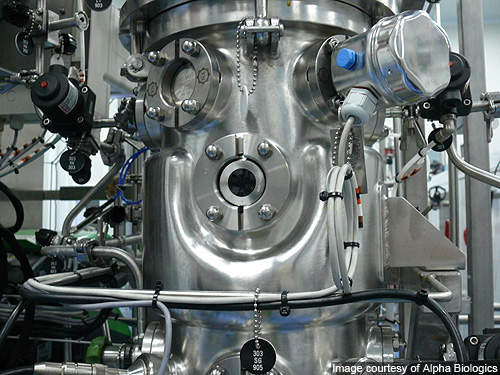
The plant spreads over an area of 5,000m² and accommodates upstream process development laboratories, cGMP manufacturing space and support areas.
The manufacturing space includes process suites, isolation suites, two independent DSP suites and separate final filtration / viral removal suites. Class 10,000 (Grade C) process suites with Class 100,000 (Grade D) support areas are encircled by a return corridor, which provides visitors an inside view through the windows.
Waste material is disposed of via the return corridor, avoiding their crossover with clean items.
There are three isolation suites used for USP-cell banking and associated operations. These suites are equipped to develop master cell banks (MCB) and working cell banks (WCB), bioreactor seed or small scale product manufacturing for pre-clinical studies.
The bioreactor suite is equipped with seven bioreactors with capacities ranging from 10l to 500l (3 x 10l, 1 x 70l, 2 x 250l, 1 x 500l). The bioreactors can be operated in batch, fed-batch or perfusion mode – and the flexible design of the facility allows utilisation of bioreactors in different combinations in a closed system. There are two recovery suites for cell harvest clarification, with medium to large scale ultra filtration capabilities.
There are two purification suites for pre- and post-virus processing, equipped with chromatography and ultra-filtration skids and dedicated cold rooms. The final filtration / viral removal suites are installed with chromatography for final filtration and bulk aliquotting.
Upstream process development laboratories are located on the ground floor of the facility and are designed to allow the scale up of cell cultures in bioreactors of different capacity. Work in the upstream process laboratories is carried out with identical equipment to the cCMP manufacturing suites.
The bioreactor suite has three bioreactors of 10l, 70l and 250l capacities, all operable on batch, fed batch and perfusion mode. Purification equipment includes both small and large scale chromatography and ultra-filtration systems.
This use of identical equipment ensures smooth technical transfer to cGMP manufacture, with a few operational changes and cleaning validation to reduce normal timelines for process development and cGMP manufacture. This, in turn, results in major cost savings for clients.
The facility also includes microbiology and quality control laboratories for environmental testing, analytical laboratories for in-process assays and a buffer preparation unit with 50l, 120l, 250l and 1000l preparation vessels.
There is also a cell bank and product storage zone for -20°C, -80°C and liquid and vapour phase nitrogen storage.
Additional equipment in this part of the plant includes process washing machines, sterilising autoclaves and CIP station.
Production
The facility is dedicated to the production of proteins secreted from mammalian cell cultures, including monoclonal antibodies and recombinant proteins. Hybridoma, CHO and NSO cell lines are the most common cell types used.
Prior to cGMP manufacturing, the facility develops cell lines and master and manufacturing working cell banks. The facility also produces supernatants, membrane fractions or other product options.
The facility has been designed to combine bioreactors with perfusion technology whenever required, to increase production capacity by 20 fold. This combination of stirred tank processes and perfusion enables ultra-efficient production of secreted protein for pre-clinical or clinical trial studies or early market use.