According to research published by The Economist Intelligence Unit[i], drugs developed using patient-centric designs have a higher chance of launch compared to drugs developed without. The research also reported that patient-centric trials take less time to recruit patients. This is crucial, as figures obtained from GlobalData’s enrolment module show that only 46% of clinical trials are meeting their enrolment target, with insufficient accrual as the leading cause of trials being withdrawn, suspended or terminated.
The data also shows that patient satisfaction with their experience during a trial is key. A positive experience by the participant reduces the likelihood of dropout, and so improves the quality of data while also reducing study costs.
With this increased awareness of the link between trial success and the patient experience, patient-centricity has risen in prevalence in recent years. However, enrolling and retaining patients can be challenging for clinical trials. Some trials have strict criteria for who can participate, which can limit the pool of eligible individuals. Access to clinical trials is limited and both geographical barriers and language differences can be a significant hurdle in recruiting diverse participants, especially for global trials.
Enhancing participant engagement
According to Daniel J Herron, Vice President of Digital Offerings, Regulated Industries at multinational translation company RWS, patient centricity in clinical trials is all about making the patient's experience a top priority.
“[Patient-centricity] means actively involving patients in the planning and design of trials, so their perspectives and needs are considered,” Herron says. “It also means making sure that the information patients receive is easy to understand.”
Diversity, equity, and inclusion (DEI) are crucial to this. As people may experience the same disease differently, it is essential to include people with a variety of lived experiences, living conditions, race, ethnicity, age, sex, and sexual orientation.[ii]
“When you design trials with the patient's viewpoint at the forefront, you foster inclusivity and accessibility,” explains Herron. “This strategy actively involves underrepresented patient groups, dismantles language barriers through precise translation, and enhances the user-friendliness of protocols and materials. It plays a pivotal role in achieving DEI targets by ensuring that all patient demographics are acknowledged and encompassed in the trial.”
A key factor is translation and use of language, says Herron:
“Miscommunication and poor translation in clinical trials can lead to serious issues. Patients may not fully understand their roles, the trial process, or potential risks and benefits. This can result in misunderstandings, errors in data reporting, and even safety concerns.”
Supporting patient engagement
Data shows that clinical studies that involve participants commuting long distances between trial sites or trials that require lots of visits, increase the patient burden. Digital technologies, such as telemedicine, remote patient monitoring, and wearable devices have been shown to reduce this burden, but not all participants are confident using technology. Research from GlobalData’s Clinical Trials database shows that the use of virtual components has drastically increased over the last decade, peaking in 2021 following the COVID-19 pandemic. In 2022, figures remained higher than in pre-pandemic years, indicating that these approaches proved successful.
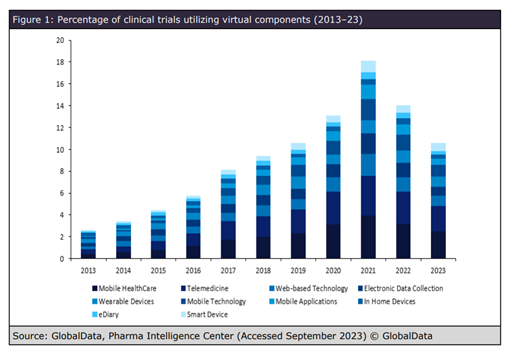
“Telemedicine will allow for remote patient visits and monitoring, making it more convenient for participants," says Herron. “Wearable devices will provide real-time data collection and monitoring, reducing the need for frequent in-person visits. These patient-centric digital platforms will improve communication and engagement and create a more patient-friendly environment, ensuring that trials are not only successful but also provide a better experience for patients.”
According to Sonnika Lamont, MRes, GlobalData analyst, Trials Intelligence: “Industry, sponsors, and sites are learning that they need to connect and align with patients to improve their experience, as the patients’ perspectives may differ from what is assumed.
“Although companies running DCTs and focusing on patient centricity should be applauded, the perpetual issue of accrual and retention across trials indicates that there is still a long way to go before true people centricity is achieved throughout all clinical trials.”
This is where RWS can help. Its translation services ensure that all trial documents are accurately translated and culturally sensitive, making it possible for patients to fully engage in the trial by providing them with clear and accessible information in their native languages, thus increasing the patient pool and reducing the instances of drop-out.
“This ultimately improves patient engagement and the overall success of the trial,” says Herron. “Patient centricity should be a fundamental approach in clinical trials because it leads to better engagement, retention, and overall success of the trial,” concludes Herron. “When patients feel valued and informed, they are more likely to stick with the trial requirements, provide high-quality data, and contribute to meaningful study outcomes. It's a win-win situation for both patients and researchers.”
For more information on how RWS translation services can help with your clinical trial, download the free paper below.
[i] https://druginnovation.eiu.com/patient-centric-trials/summary/patient-centric-trials-summary/
[ii] https://www.nimhd.nih.gov/resources/understanding-health-disparities/diversity-and-inclusion-in-clinical-trials.html









