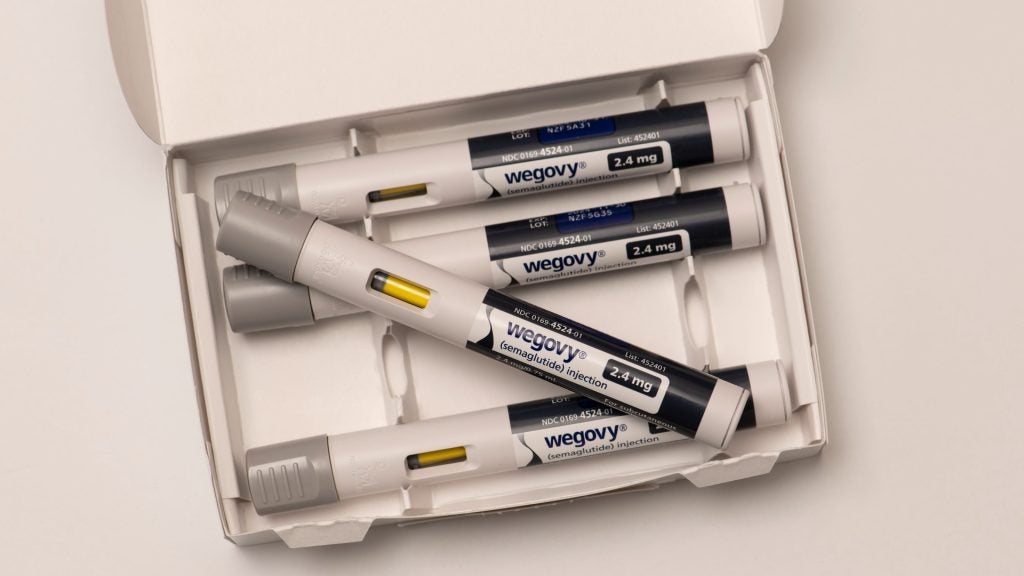
Novo Nordisk’s blockbuster weight-loss drug Wegovy may reduce the risk of heart attack before the weight loss effects take place, the company revealed on Saturday (11 November).
In a study of 17,604 patients spanning four and half years, results indicate that it cuts major cardiovascular risks by 20% in patients with obesity and heart disease but without diabetes. In a press conference before the American Heart Association annual scientific meeting, Novo suggested that as weight loss had not begun when the cardiovascular benefits first appeared, the drug’s benefits were not purely due to a reduced body mass index (BMI).
Regardless of the mechanism, the results are impressive. The study, released in the New England Journal of Medicine (NEJM), notes that “the concept of treating obesity to reduce the risk of cardiovascular complications has been hampered by the lack of evidence from trials indicating that lifestyle or pharmacologic interventions for overweight or obesity improve cardiovascular outcomes.”
By proving that Wegovy has major implications for heart disease patients, Novo has paved the way for a label expansion that it has pursued since August. Novo's stock opened at 2.22% higher today from Friday’s close. However, the excitement comes with a few key caveats.
Most importantly, it is unknown whether the drug has preventative effects on cardiovascular disease, given that all trial patients were already diagnosed with heart conditions. In addition to increasing safety for those with pre-existing conditions, if the drug could be shown to reduce the risk of getting heart disease, the boon would be far greater.
Additionally, the risk of death from cardiovascular causes was reduced by 15%, which is below the statistical significance threshold for the study. Also, twice as many patients on Wegovy exited the trial early due to adverse events (16.6%) as those on placebo (8.2%). The gastrointestinal effects of Wegovy were the main culprit, and they remain a cause for concern as usage of the drug proliferates.
The competitors
Despite all of this, the biggest worry for Novo Nordisk remains its competitors. Last week (9 November) the FDA approved Eli Lilly’s weight loss drug Zepbound (trizepatide), which will be a major competitor to Wegovy. It is also a GLP-1RA, meaning that it operates on the same active mechanism as semaglutide marketed as Wegovy and Ozempic) but boasts a potential for even greater weight loss according to clinical trials.
The same day, AstraZeneca announced a licencing agreement with clinical-stage biotech Eccogene to develop a small molecule GLP-1RA that can be taken orally rather than subcutaneously. Wegovy already transformed the weight loss market by only requiring weekly injections rather than the daily ones required by its predecessors, so removing it altogether could be another game changer.
By releasing the results first, Novo has a chance to stay ahead of its competitors by securing the first expansion into the cardiovascular health market. But the lead is closing fast. Whilst data is not yet available for expected sales of Zepbound, its diabetes-treating sister Mounjaro is due to overtake Ozempic in sales by 2027 according to GlobalData estimates. By 2029 sales for Mounjaro are expected to reach $27bn compared to only $17.8bn for Ozempic.
GlobalData is the parent company of Pharmaceutical Technology.
Novo also faces the problem of many market leaders: it takes the risk, but its competitors may well benefit from the results of this study. The Wegovy trial data may also spur off-label prescription of other drugs in the same class like Zepbound, in the meantime, while Lilly and other companies gather data from their own cardiovascular studies.
Our signals coverage is powered by GlobalData’s Thematic Engine, which tags millions of data items across six alternative datasets — patents, jobs, deals, company filings, social media mentions and news — to themes, sectors and companies. These signals enhance our predictive capabilities, helping us to identify the most disruptive threats across each of the sectors we cover and the companies best placed to succeed.