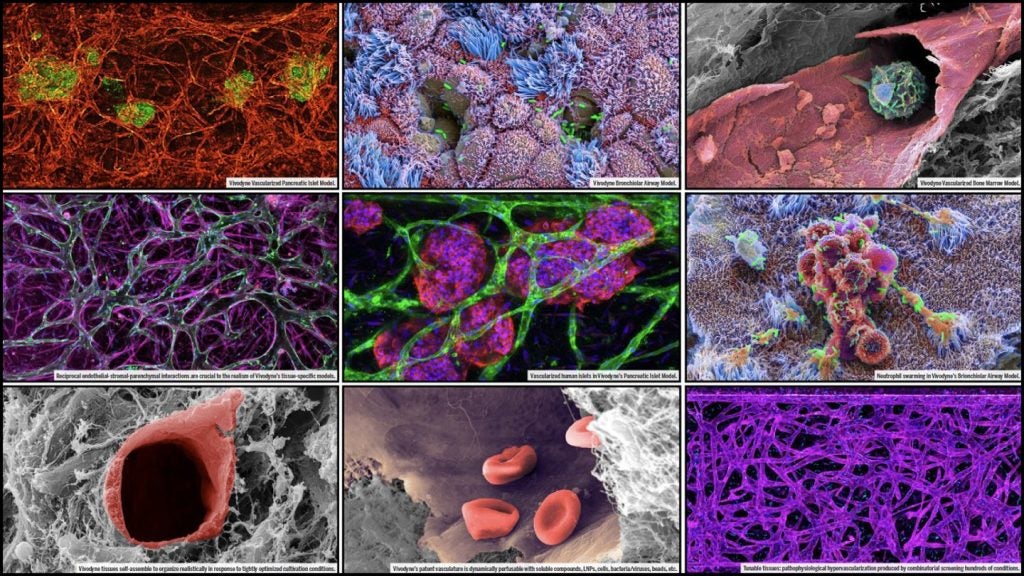
Biotech company Vivodyne has raised $38m in total seed financing for the development of lab-grown human organs.
Led by Khosla Ventures, the financing round saw participation from CS Ventures, Bison Ventures, MBX Capital and Kairos Ventures.
Vivodyne will use the funding to advance its discovery pipeline and clinically predictive AI stack.
This AI stack will detect new therapeutic targets and predict the responses of patients to new drugs by testing directly on lab-grown human organ tissues.
The company has bio-engineered more than 20 human organ tissue types that mimic native human physiology and can precisely capture new therapy effects and predict patient outcomes at a tissue, cellular, organ and systemic scale.
Vivodyne's innovative method for drug discovery generates patient-level human results before clinical trial testing of a drug.
The company's platform has been developed to cultivate, dose and analyse more than 10,000 individual human tissues through robotic automation.
This enables the generation of extensive human datasets, which will drive the next generation of human-trained AI for drug discovery.
Vivodyne CEO and co-founder Andrei Georgescu stated: “By combining the principles of organoids and organs-on-chips, we’ve created a new class of lifelike, lab-grown human organs.
“We use these lab-grown human test subjects to discover and develop new therapies for human diseases.
“The result is huge amounts of complex human data — larger than you could get from any clinical trial — and we train multimodal models on this data to predict and improve the safety and efficacy of new drugs.”
Leveraging its AI platform, Vivodyne has designed and enhanced modalities of drugs, including small molecules, biologics, antibody-drug conjugates, antibodies to mRNA-bearing lipid nanoparticles and cell therapies.